A xanthene polyurethane hapten, artificial antigen and application thereof
A technology of polyurethane and artificial antigen, which is applied in the field of immunoassay, can solve problems such as unestablished, and achieve the effects of enhanced structural features, strong specificity, and high derivation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 Urethane Hapten B Ⅰ (n=0) Preparation method
[0047] 178mg of ethyl carbamate was dissolved in 5mL of 1M acetic acid; 626mg of 9-hydroxy-9H-xanthene-3-carboxylic acid was dissolved in 20mL of methanol; the above solution was mixed and stirred at room temperature for 1 hour. Add 10 mL of distilled water to the mixture, remove the methanol in the reaction mixture by rotary evaporation, filter, wash the precipitate with 0.1M dilute hydrochloric acid, wash with distilled water, and dry to obtain 424 mg of gray solid, namely the target hapten XEC-313-3, the structural formula is as follows (Ⅳ) shown.
[0048]
[0049] Formula (Ⅳ).
[0050] ESI-MS analysis (negative)m / z 312[M+H] – ; 1 H NMR (600MHz, DMSO) δ8.21(d, J=8.8Hz, 1H), 7.71(dd, J=8.0, 1.6Hz, 1H), 7.61(d, J=1.6Hz, 1H), 7.51(d ,J=8.0Hz,1H),7.40(d,J=7.3Hz,1H),7.35(td,J=7.9,1.5Hz,1H),7.18(t,J=7.4Hz,3H),5.99(d ,J=8.6Hz,1H),4.06(q,J=7.0Hz,2H),1.18(t,J=7.1Hz,3H).
Embodiment 2
[0051] Example 2 Urethane Hapten B Ⅰ (n=1) preparation method
[0052]178mg of ethyl carbamate was dissolved in 5mL of 1M acetic acid; 654mg of 2-(9-hydroxy-9H-xanthene-4-yl)acetic acid was dissolved in 20mL of methanol; the mixture was mixed and stirred at room temperature for 1 hour. Add 10 mL of distilled water to the mixed solution, remove the methanol in the reaction mixed solution by rotary evaporation, filter, wash the precipitate with 0.1M dilute hydrochloric acid, wash with distilled water, and dry to obtain 402 mg of gray solid, namely the target hapten XEC-327-4, the structural formula is as follows (Ⅴ) shown.
[0053]
[0054] Formula (Ⅴ).
[0055] ESI-MS analysis (positive) m / z 350[M+Na] + ; 1 H NMR (600MHz, DMSO) δ8.09(d, J=9.0Hz, 1H), 7.36(d, J=7.6Hz, 1H), 7.30(t, J=7.2Hz, 1H), 7.21(dd, J =17.5,7.4Hz,2H),7.12(dd,J=13.9,7.1Hz,2H),7.02(t,J=7.5Hz,1H),5.92(d,J=8.9Hz,1H),4.06(q , J=7.1Hz, 2H), 3.52(dd, J=39.5, 15.3Hz, 2H), 1.18(t, J=7.1Hz, 3H).
Embodiment 3
[0056] The preparation method of embodiment 3 immunogen coating original
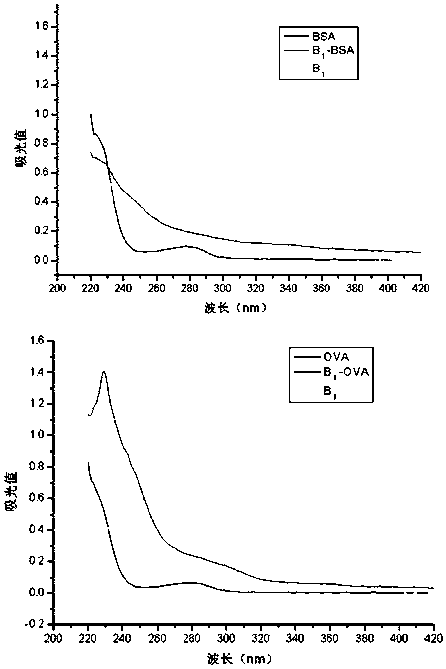
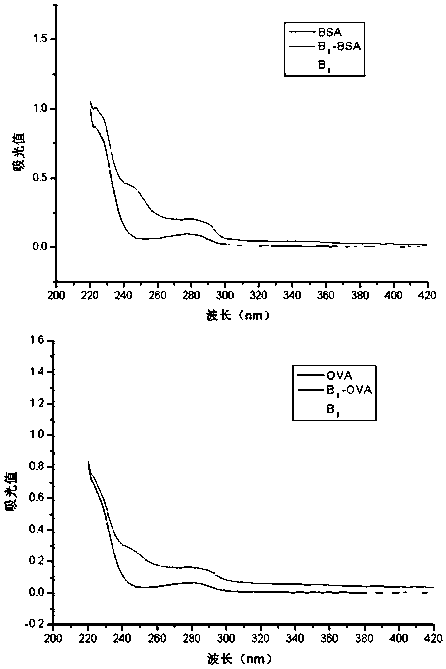
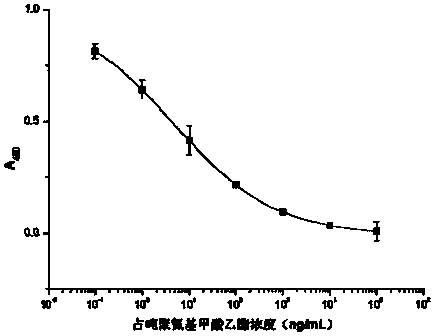
[0057] The difference between the preparation of the immunogen and the coating source lies in the carrier protein, the immunogen carrier protein uses bovine serum albumin (BSA), and the coating original carrier protein uses chicken ovalbumin (OVA). The preparation method of the immunogen is used as an example in the following.
[0058] Active ester method: take hapten B Ⅰ (n=0) 31.3mg (0.1mmoL) or hapten B Ⅰ (n=1) 32.7mg (0.1mmoL) was dissolved in 0.5mL DMF, stirred and added 25.6mg (0.1mmol) DCC and 11.5mg (0.1mmoL) NHS, magnetically stirred at 4°C overnight, the supernatant after centrifugation was A liquid. Weigh 20 mg of BSA or OVA and dissolve in 2 mL of PBS (pH 8.0) with a concentration of 0.1 mol / L, stir and dissolve to prepare liquid B. Under magnetic stirring, solution A was gradually dropped into solution B, and reacted at 4°C for 12 hours. After centrifugation, the supernatant was taken,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com