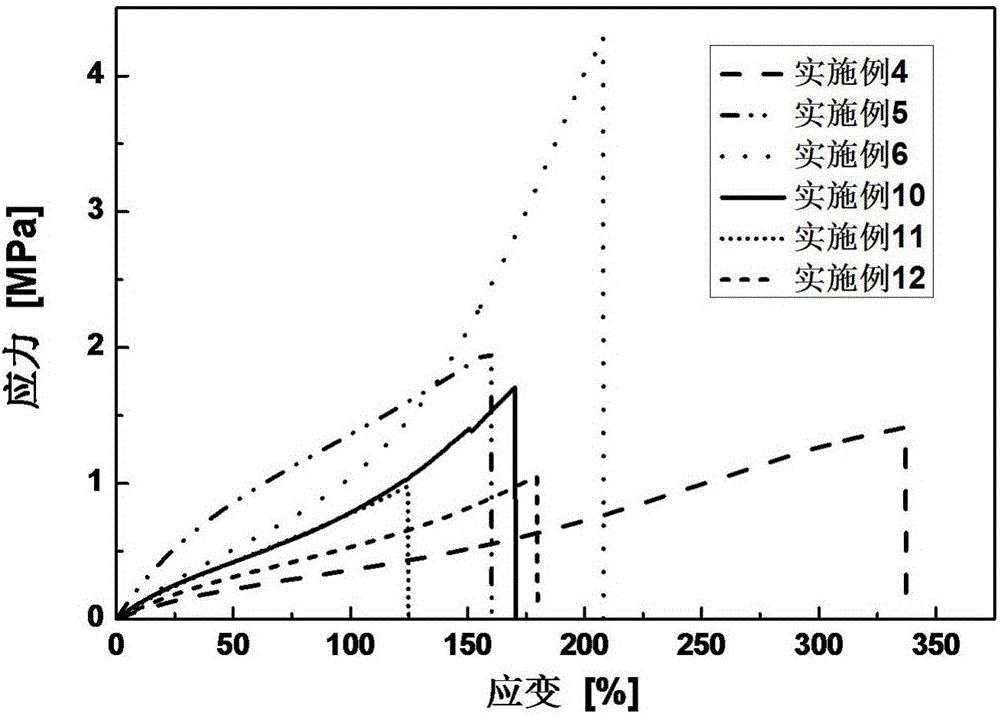
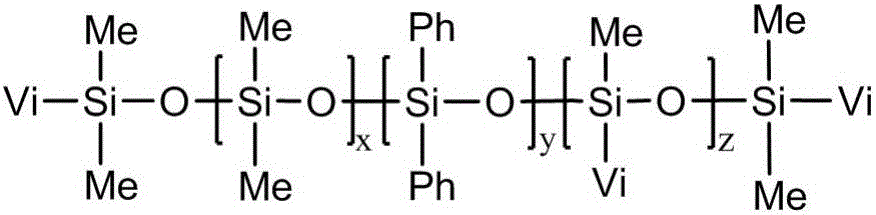Photocured organosilicone elastomer and preparation method and application thereof
A technology of elastomer and silicone, which is applied in the field of light-cured silicone elastomer and its preparation, can solve the problems of poor mechanical properties of light-cured elastomers, poor mechanical properties of transparent materials, and affecting the optical properties of materials, etc. Effect of curing rate, increasing crosslink density and mechanical strength, improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 vinylmethylphenyl polysiloxane
[0037] 30g divinyltetramethyldisiloxane, 50g tetramethyltetravinylcyclotetrasiloxane, 100g octamethylcyclotetrasiloxane and 30g octaphenylcyclotetrasiloxane were added to the flask, and then Then add 0.08% tetramethylammonium hydroxide of the total amount of the above four substances, perform ring-opening polymerization reaction at 110°C for 4 hours, then raise the temperature to 180°C to decompose the catalyst, and vacuumize to remove the low-boiling point substances to obtain Vinylmethylphenylpolysiloxane, the yield was 93.21%.
[0038] Gained vinylmethylphenylpolysiloxane molecular structural formula is as follows:
[0039]
[0040] Wherein, x:y:z=136:16:60.
Embodiment 2
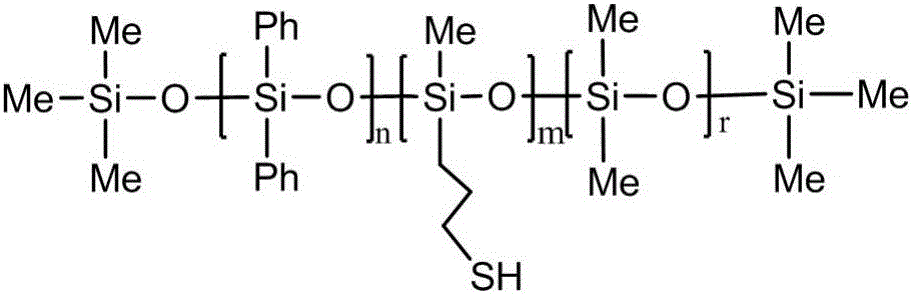
[0041] The preparation of embodiment 2 mercaptopropylmethylphenylpolysiloxane
[0042] Add 30g of hexamethyldisiloxane, 30g of mercaptopropylmethyldiethoxysilane, 75g of diphenyldiethoxysilane, and 90g of dimethyldiethoxysilane into a three-necked flask, and then use hydrochloric acid to The pH of the reaction system was adjusted to 5, the hydrolysis-condensation reaction was carried out at 70° C. for 3 hours, and the water and solvent were distilled off to obtain mercaptopropylmethylphenylpolysiloxane with a yield of 89.74%.
[0043] Gained mercaptopropylmethylphenylpolysiloxane molecular structural formula is as follows:
[0044]
[0045] Wherein, n:m:r=27:17:61.
Embodiment 3
[0046] Preparation of embodiment 3 mercaptopropyl silicone resin
[0047] Add 30g of hexamethyldisiloxane, 150g of mercaptopropyltriethoxysilane, and 75g of phenyltriethoxysilane into a three-necked flask, then add hydrochloric acid to control the pH of the reaction system at 4.5, and then Co-hydrolysis-polycondensation reaction was carried out for 5 hours, and finally the temperature was raised to 120° C. to remove low boilers by vacuuming to obtain mercaptopropyl silicone resin with a yield of 83.27%.
[0048] The molar ratio of the mercaptopropyl segment, the phenyl segment and the trimethyl silicon segment in the obtained mercaptopropyl silicone resin molecule is 0.63:0.3:0.37.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com