Method for recycling nickel, cobalt and manganese synchronously from waste residues containing nickel, cobalt and manganese
A nickel-cobalt-manganese and waste slag technology, which is applied in the field of environmental protection and resource recycling, and can solve the problem of high cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
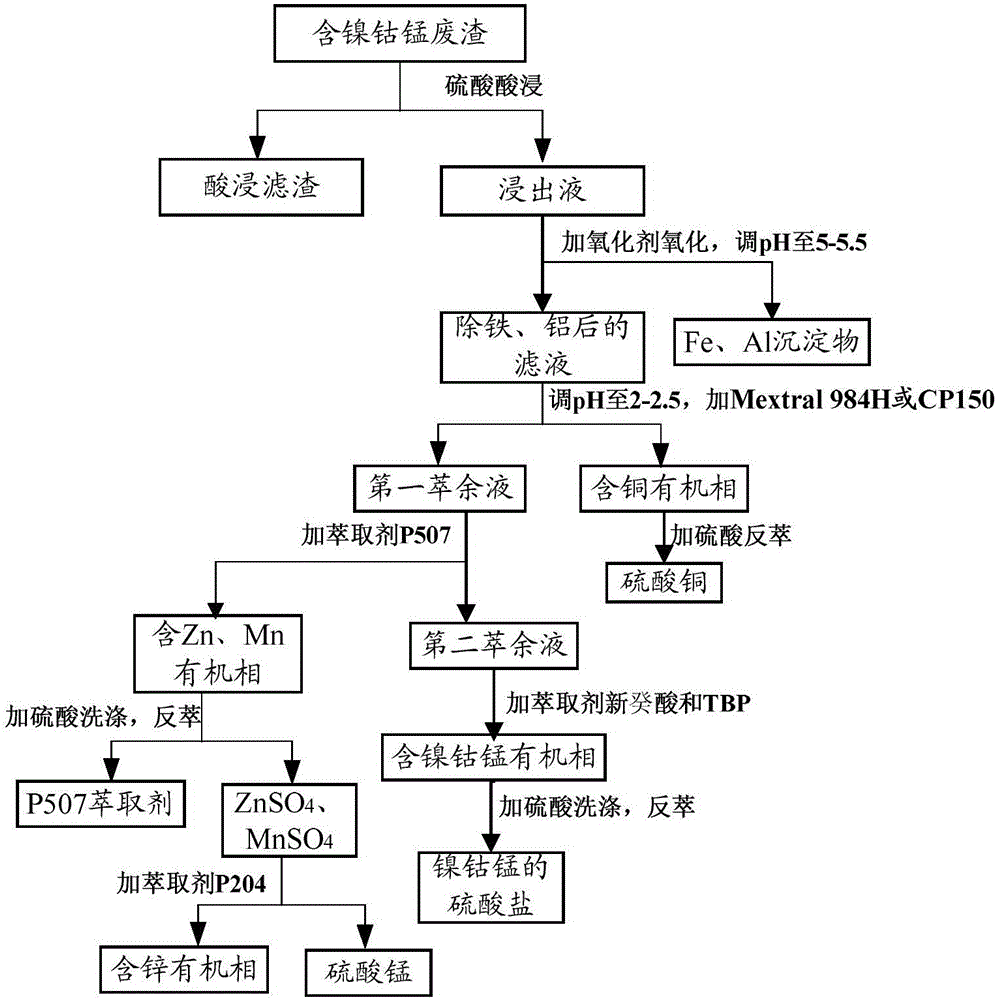
[0066] See figure 1 In the process flow diagram, a method for synchronously reclaiming nickel-cobalt-manganese from nickel-cobalt-manganese waste slag is provided in the present embodiment one, comprising the following steps:
[0067] (1) Sulfuric acid leaching: take the waste residue containing nickel, cobalt and manganese, which comes from waste batteries, manganese liquid purification residue, low-grade laterite ore and other mixtures; add the mass fraction according to the solid-liquid ratio of 100:1g / L Carry out acid leaching for 30% sulfuric acid solution, the temperature of acid leaching is 50°C, the time of acid leaching is 30min, the pH at the end of leaching is controlled to be 1.0, the solid and liquid are separated by filtration, and the leachate is collected;
[0068] The concentrations of main components in the leachate are: Ni 2.84g / L, Co 40.8g / L, Mg 12.7g / L, Mn7.2g / L, Fe 1.53g / L, Al 93mg / L, Cu 770mg / L, Ca 547mg / L, Zn 631mg / L;
[0069] (2) Iron removal, alumi...
Embodiment 2
[0078] A method for synchronously recovering nickel, cobalt and manganese from sulfuric acid leaching solution containing nickel, cobalt and manganese waste residue, comprising the following steps:
[0079] (1) Sulfuric acid leaching: take the waste residue containing nickel, cobalt and manganese, add a mass fraction of 25% sulfuric acid solution for acid leaching according to the solid-liquid ratio of 120:1g / L, the temperature of the acid leaching is 60°C, and the time of acid leaching is 35min, control the pH at the end of the leaching to 0.5, filter and separate the solid and liquid, and collect the leachate;
[0080] The concentrations of the main components in the leachate are: Ni 5.23g / L, Co 18.5g / L, Mg 6.5g / L, Mn8.2g / L, Fe 2.19g / L, Al 147mg / L, Cu 1370mg / L, Ca 416mg / L, Zn 330mg / L;
[0081] (2) Iron removal, aluminum: get above-mentioned leaching solution, add oxidant sodium chlorate (being n(NaClO 3 )=0.42n(Fe 2+ )), adjust the pH=5.3 with NaOH, age at a temperature o...
Embodiment 3
[0090] A method for synchronously recovering nickel, cobalt and manganese from sulfuric acid leaching solution containing nickel, cobalt and manganese waste residue, comprising the following steps:
[0091] (1) Sulfuric acid leaching: take the waste residue containing nickel, cobalt and manganese, add a sulfuric acid solution with a mass fraction of 20% according to the solid-liquid ratio of 150:1g / L for acid leaching, the temperature of the acid leaching is 80°C, and the time of acid leaching is 15min , control the pH at the end of leaching to be 1.2, filter and separate the solid and liquid, and collect the leachate;
[0092] The concentrations of main components in the leachate are: Ni 5.12g / L, Co 27.3g / L, Mg 9.9g / L, Mn10.4g / L, Fe 1.87g / L, Al 165mg / L, Cu 965mg / L, Ca 732mg / L, Zn 1030mg / L;
[0093] (2) Iron removal, aluminum: get above-mentioned leaching solution, add oxidizing agent sodium hypochlorite (being n(NaClO)=0.75n(Fe 2+ )), adjust pH=5.0 with NaOH, age at a tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com