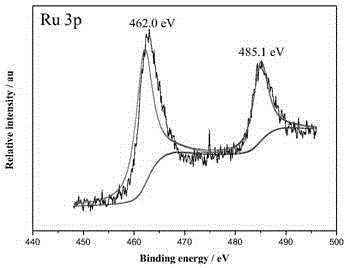
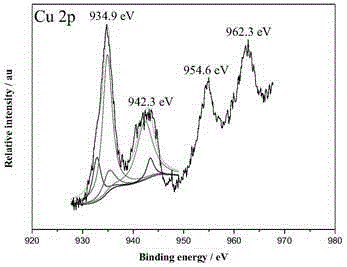
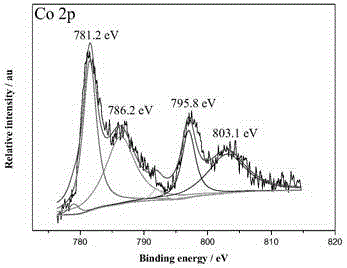
Ternary nanometer catalyst used for hydrolyzing ammonia borane to release hydrogen and preparation method of ternary nanometer catalyst
A nano-catalyst, ammonia borane technology, applied in the field of hydrogen storage materials, can solve the problems of large active component particles, easy sintering and aggregation, easy aggregation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of ternary RuCuCo@MIL-101 nanocatalyst
[0024] Weigh terephthalic acid (823.0 mg, 5.0 mmol), Cr(NO 3 ) 3 9H 2 O (2007.0 mg, 5.0 mmol), HF (0.12 mL, 40 wt%) and deionized water (24 mL) were stirred and added to a 50 mL stainless steel reactor with a polytetrafluoroethylene liner, sealed, and kept at a constant temperature of 200 °C Under reaction 8 h. After cooling to room temperature, a green suspension was obtained, which was filtered through a filter cloth with a pore size of 100 um. Then the filtrate containing MIL-101 particles was suction filtered, washed with deionized water, dried, stirred in ethanol solution at 70ºC for 6h and NH 4 F (30 mM) solution was stirred at 60ºC for 6h, filtered and dried to obtain MIL-101.
[0025] Weigh 200.0 mg of the above MIL-101, 29.0 mgCu(NO 3 ) 2 •3H 2 O, 35.0 mg of Co(NO 3 ) 2 •6H 2 O and 6 mL 0.01 M RuCl 3 Add it into 30 mL deionized water, sonicate for 10 min to obtain a uniformly dispersed ...
Embodiment 2
[0027]Example 2: Preparation of one-component Ru@MIL-101 catalyst
[0028] Weigh 200.0 mg of MIL-101 from Example 1 above and 6 mL of 0.01 M RuCl 3 Add it into 30 mL deionized water, sonicate for 10 min to obtain a uniformly dispersed suspension and continue stirring for 24 h. Then, weigh 50.0 mg of NaBH 4 The solid was dissolved in 10 mL of deionized water, and the solution was added dropwise to the above suspension to reduce the metal ions in the solution, and stirring was continued for 6 h after the dropwise addition was completed. The Ru@MIL-101 catalyst was obtained after the product was filtered, washed, and vacuum-dried overnight. The average particle size of the supported metal Ru was measured by TEM to be 1.9nm.
Embodiment 3
[0029] Example 3: Preparation of binary CuCo@MIL-101 catalyst
[0030] Weigh 200.0 mg of the MIL-101 of the above-mentioned Example 1, 29.0 mg of Cu(NO 3 ) 2 •3H 2 O and 35.0 mg of Co(NO 3 ) 2 •6H 2 O was added to 30 mL of deionized water, sonicated for 10 min to obtain a uniformly dispersed suspension, and then continued to stir for 24 h. Then, weigh 50.0 mg of NaBH 4 The solid was dissolved in 10 mL of deionized water, and the solution was added dropwise to the above suspension to reduce the metal ions in the solution, and stirring was continued for 6 h after the dropwise addition was completed. The CuCo@MIL-101 catalyst was obtained after the product was filtered, washed and vacuum dried overnight. The average particle diameter of supported CuCo metal is 7.8nm as measured by TEM.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com