Brucella fluorescence polarization (FPA) detection kit
A detection kit, Brucella technology, applied in the direction of measuring devices, immunoassays, instruments, etc., can solve problems such as difficult to cure, animal reproduction and production performance hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
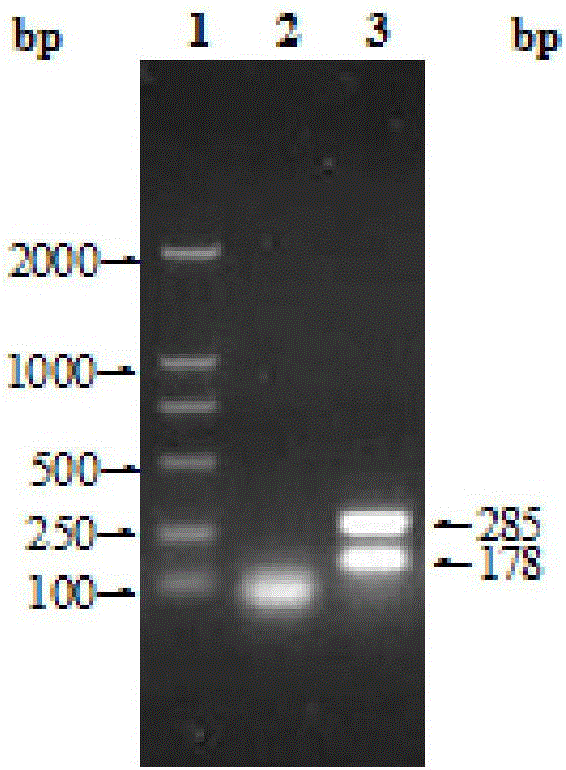
[0037] ——The quality standard of the bacterial species (B. suis S2 strain, B. suis S2) used for antigen preparation.
[0038] 1. Colony shape The edges of the colony are neat, round, dewdrop-shaped, irradiated by oblique light, and slightly blue opalescent when observed against the light.
[0039] 2. The staining form is cocci, scattered individually, without forming spores and capsules. The size is between 0.6 and 2.5 μm. Gram's stain was negative, Korotkoff's stain was red.
[0040] 3. The pure inspection is carried out according to the appendix of the existing "Chinese Veterinary Pharmacopoeia" (Chinese Veterinary Pharmacopoeia Committee, the People's Republic of China Veterinary Pharmacopoeia, 2010 edition three, China Agricultural Press, 2011, hereinafter referred to as "Chinese Veterinary Pharmacopoeia"), should purely.
[0041] 4. Specificity test
[0042] (1) Serological specificity Antigens made from cultures should agglutinate with smooth-type Brucella-positive s...
Embodiment 2
[0053] ——Purification and labeling method of the antigen OPS used in the detection of this application
[0054] 1. Bacterial suspension preparation Inoculate the qualified secondary seeds of B. suisS2 strain in pancreas agar flat bottles, and after culturing at 37°C for 48 hours, add 20ml of normal saline containing 0.5% phenol to each bottle to soak the surface of the bacteria for more than 10 minutes , wash the culture and collect in sterile glass bottles.
[0055] 2. Bacterial liquid inactivation and inactivation test Heat the collected bacterial suspension to 80°C for 120 minutes, and store it at 4°C after cooling naturally. The inactivation test should be carried out on the inactivated bacteria solution. Take 0.1ml of the inactivated bacteria solution and spread it on pancreatic agar or TSA plate, culture it at 37°C for 7 days, and it should not grow sterilely.
[0056] 3. Purification of OPS Centrifuge the bacterial suspension that passed the inactivation test at 10,00...
Embodiment 3
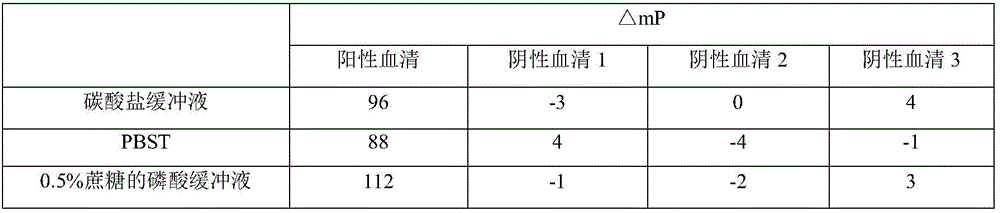
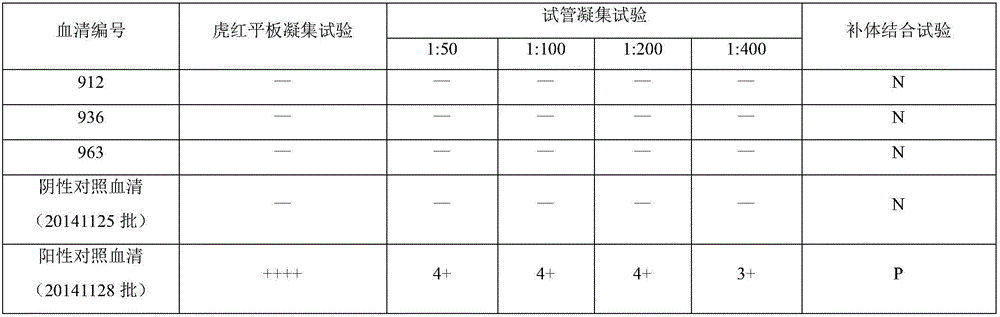
[0059] ——Preparation and inspection standards of negative control serum and positive control serum in the kit of this application
[0060] 1. Preparation of Negative Control Serum
[0061] (1) Animals are about 2-year-old, sexually mature healthy cattle that are negative for Brucella antibodies by tiger bengal plate agglutination test, test tube agglutination test and complement fixation test.
[0062] (2) Serum preparation Blood was collected from the jugular vein, the serum was separated, sterilized by filtration with a 0.45 μm filter, the collected serum was added with 0.05% proclin300 for preservation, and aseptically packaged, and after passing the inspection, it was stored at -20°C for later use.
[0063] 2. Test of negative control serum
[0064] (1) Appearance Orange or light yellow clear liquid.
[0065] (2) Sterility test is carried out according to the appendix of the current "Chinese Veterinary Drug Code", and the growth should be sterile.
[0066] (3) Specifici...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com