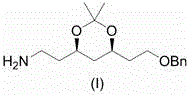
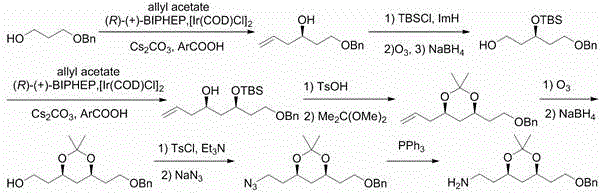
Preparation method of atorvastatin measuring chain intermediate
A technology of reaction time and organic solvent, applied in the direction of organic chemistry, etc., can solve the problems of unsuitable industrial production, limited industrial application, inability to recycle, etc., and achieve the effect of mild reaction conditions and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
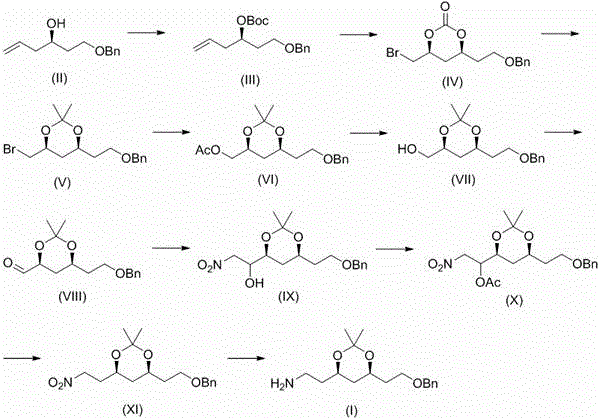
[0049] Embodiment 1, tert-butyl ( R )-1-benzyloxyhex-5-en-3-yl carbonate (III) preparation
[0050] Will( R )-1-benzyloxyhex-5-enol (II) (100 g, 485 mmol), dichloromethane (500 mL), zinc acetate (9 g, 4.9 mmol) and di-tert-butyl dicarbonate (127 g, 582 mmol) were placed In the reaction flask, heat and stir to reflux for 12 h, after the reaction is completed, cool to room temperature, filter, and concentrate the filtrate with solvent, and then distill under reduced pressure to obtain a pale yellow oily liquid (III) (134 g, 91%).
Embodiment 2
[0051] Embodiment 2, tert-butyl ( R )-1-benzyloxyhex-5-en-3-yl carbonate (III) preparation
[0052] Will( R )-1-benzyloxyhex-5-enol (200g, 0.97mol), toluene (1L), zinc acetate (18g, 9.8mmol) and di-tert-butyl dicarbonate (233g, 1.07mol) were placed in a reaction flask , heated and stirred at 80 °C for 6 h, the reaction was completed, cooled to room temperature, filtered, the filtrate was concentrated to solvent, and then distilled under reduced pressure to obtain a pale yellow oily liquid (III) (279 g, 94%).
Embodiment 3
[0053] Embodiment 3, (4 S ,6 S )-4-(2-benzyloxyethyl)-6-bromomethyl-2-oxo-1,3-dioxane (IV) preparation
[0054] Compound (III) (50 g, 163 mmol), potassium carbonate (34 g, 244 mmol) and dichloromethane (500 mL) were placed in a dry reaction flask, and bromine (31 g, 196 mmol) was added dropwise at -40°C with stirring. The dripping was completed within 0.5 h, and the mixture was kept under stirring for 1 h. After the reaction was completed, it was quenched with 5% sodium bisulfite solution, and the layers were separated. The aqueous phase was extracted with dichloromethane, and several layers were combined, washed with water, dried over anhydrous sodium sulfate, and filtered. , the filtrate was concentrated to give a colorless oily liquid (IV) (52.6g, 98%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com