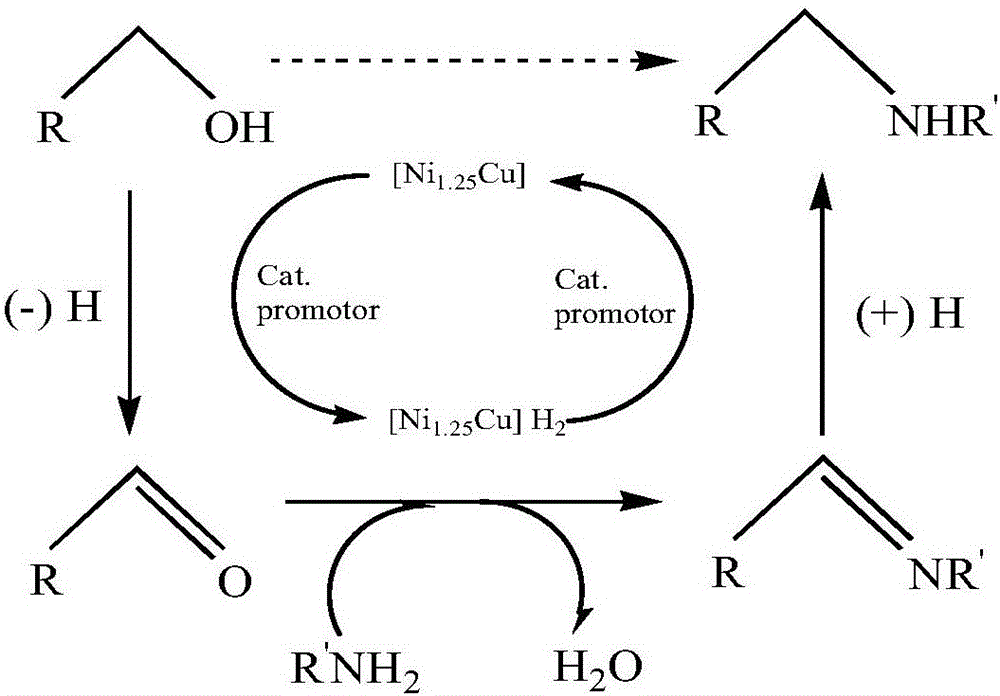
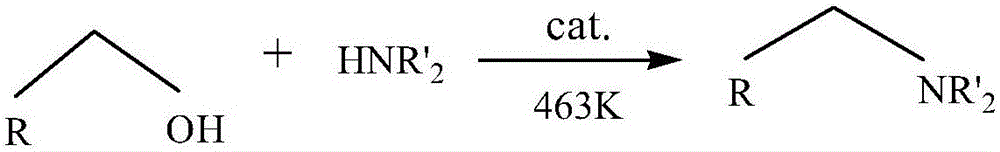Catalyst used for catalyzing synthesis of aliphatic tertiary amine from primary amine or secondary amine and fatty alcohol as well as preparation and application of catalyst
A technique for synthesizing fatty alcohols and catalyzing primary amines, which is used in the field of catalyzing the synthesis of fatty tertiary amines from primary or secondary amines and fatty alcohols. It can solve the problem of declining economic benefits of alkyl fatty tertiary amines and raising industrial production costs by precious and rare metal elements. and other problems, to achieve the effect of easy-to-obtain raw materials, low cost, easy operation, and no need for solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Catalyst preparation:
[0034] Mix and dissolve a calculated amount of required metal nitrate solid and a certain amount of deionized water in a beaker, heat to 323K and magnetically stir to dissolve completely. Co-precipitation method is adopted in the process of preparing the catalyst, and the precipitation agent is NaOH+Na 2 CO 3, the molar ratio is 1:1, and the mass fraction is about 10%. First, the precipitant is evenly pumped into the beaker containing the aluminum nitrate solution by a peristaltic pump at an initial flow rate of 5 mL / min, the magnetic stirring speed is 5 r / s, and the temperature in the beaker is raised to 353 K, so that the aluminum nitrate solution is finally completely converted into Aluminum hydroxide gel, and then add the nitrate solution of the active components in the order of Ni, Mg, Cu, Fe for co-precipitation, and maintain the pH value of the solution at about 9 by adjusting the flow rate pumped by the peristaltic pump. After the drop...
Embodiment 2
[0038] Alkylation of primary amines
[0039]
[0040] Add n-propylamine (0.15mol) and n-octanol (0.6mol) to a 300mL three-necked flask in sequence, add 0.02mol catalyst, feed hydrogen at a flow rate of 0.2L / min, and heat to 190°C for 7h. Samples were taken every hour, and the product was separated and purified by column chromatography. GC-MS detected that the yield of aliphatic tertiary amine increased with the increase of reaction time, reaching a maximum of 96.35% at the 6th hour, and the conversion rate and selectivity were 97.56% respectively. and 98.75%, and then the yield remained stable. The catalyst prepared by Example 1, the components and proportioning ratio are: Ni:Cu:Mg:Fe=1.25:1:0.3:0.6, and its mass fractions are respectively: nickel: 21.75%; copper: 18.95%; magnesium: 4.25% %; Iron: 6.32%.
[0041] After the reaction, filter, wash and dry to obtain the reacted catalyst. After using this catalyst to participate in the reaction repeatedly for 4 times, it can...
Embodiment 3
[0043] Alkylation of secondary amines
[0044]
[0045] Add di(n-propyl)amine (0.15mol) and n-octanol (0.3mmol) successively into a 300mL three-necked flask, add 0.02mol of catalyst, feed hydrogen at a flow rate of 0.2L / min, and heat to 190°C for 7h. Samples were taken every hour, and the product was separated and purified by column chromatography. GC-MS detected that the yield of aliphatic tertiary amine increased with the increase of reaction time, reaching a maximum of 97.33% at the 6th hour, and the conversion rate and selectivity were 98.56% respectively. and 98.75%, and then the yield remained stable. The catalyst prepared by Example 1, the components and the proportioning ratio are: Ni:Cu:Mg:Fe=1.25:1:0.3:0.6, and its mass fractions are respectively: nickel: 21.75%; copper: 18.95%; magnesium: 4.25% %; Iron: 6.32%.
[0046] After the reaction, filter, wash and dry to obtain the reacted catalyst. After using the catalyst to repeatedly participate in the reaction for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com