Method for preparing acrivastine
A technology of avastatin and avastin, which is applied in the field of drug synthesis, can solve the problems of cumbersome operation steps, large amount of three wastes, and increased production costs, and achieve the effects of high purity, reduced operating hours, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
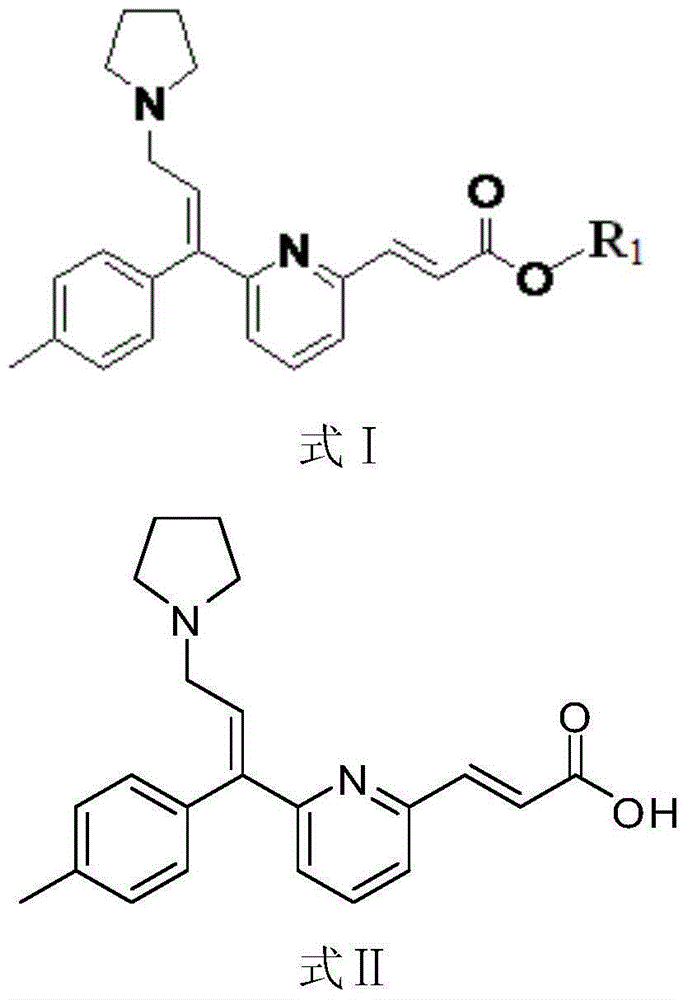
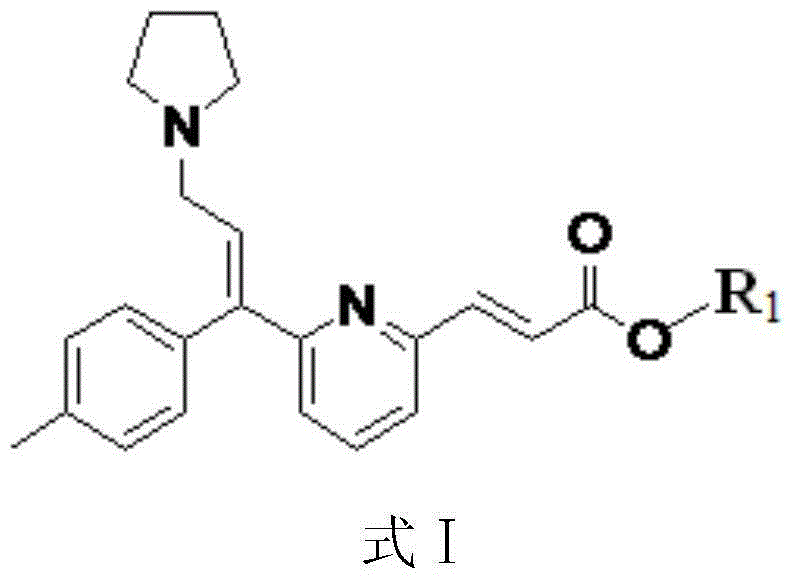
[0036] In a 100ml three-neck flask equipped with a stirrer and a thermometer, add 50g of anhydrous methanol, start stirring, then add 8.0g of ethyl arvalate, and protect with nitrogen. Heat up to 35°C-45°C to dissolve, then add 0.8g of activated carbon, keep warm for half an hour for decolorization. After filtering, the filtrate was transferred to a three-necked flask, and 26.5 g of 6.7% potassium hydroxide solution was added dropwise. Keeping the temperature at 35°C-45°C, after reacting for 1.0h, TLC spot plate (dichloromethane / methanol=9 / 1) showed that the reaction was complete. Concentrate under reduced pressure until the flow is cut off, add 8 g of purified water, cool down to 20°C-30°C, adjust the pH value to 5.0 with 6% sulfuric acid solution, stir for 10 minutes and repeat the test. Cool down to 5°C-15°C in an ice bath, and crystallize for 4 hours. Suction filtration, rinse the filter cake with purified water 8g×2, suction filtration, and dry the filter cake at 90°C u...
Embodiment 2
[0038]In a 100ml three-necked flask equipped with a stirrer and a thermometer, add 50g of absolute ethanol, start stirring, then add 8.0g of ethyl arvalate, and protect with nitrogen. Heat up to 35°C-45°C to dissolve, then add 2.0g of silica gel, keep warm for half an hour for decolorization. After filtering, the filtrate was transferred to a three-necked flask, and 28.4 g of 12% sodium carbonate solution was added dropwise. Raise the temperature to 60°C, react for 2 hours, TLC spot plate (dichloromethane / methanol=9 / 1) shows that the reaction is complete, concentrate under reduced pressure until the flow is cut off, add 16g of purified water, cool down to 20°C-30°C, and use 6% phosphoric acid The pH value of the solution was adjusted to 6.0, stirred for 10 minutes and retested. Cool down to 0°C-10°C in an ice bath, and crystallize for 3 hours. After suction filtration, the filter cake was rinsed with 16 g of purified water × 2, suction filtered, and the filter cake was dried...
Embodiment 3
[0040] In a 100ml three-necked flask equipped with a stirrer and a thermometer, add 50g of absolute ethanol, start stirring, then add 8g of ethyl avalate, and protect with nitrogen. Heat up to 35°C-45°C to dissolve, then add 0.8g of activated carbon, keep warm for half an hour for decolorization. After filtration, 26.5 g of 5% sodium hydroxide solution was added dropwise to the filtrate. Keeping the temperature at 35°C-45°C, after reacting for 1.0h, TLC spot plate (dichloromethane / methanol=9 / 1) showed that the reaction was complete. Concentrate under reduced pressure until the flow is cut off, add 8 g of purified water, cool down to 20°C-30°C, adjust the pH value to 6.5 with 6% sulfuric acid solution, stir for 10 minutes and repeat the test. Cool down to 0°C-10°C in an ice bath, and crystallize for 6 hours. After suction filtration, the filter cake was rinsed with purified water 8g×2, suction filtered, and the filter cake was dried under reduced pressure at 90°C to obtain 6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com