Preparing method for 1,1-cyclopropyl dimethyl carbinol
A technology of cyclopropyl dimethanol and diethyl cyclopropyl dicarboxylate, which is applied in the field of pharmaceutical synthesis, can solve the problems of harsh reaction conditions, low environmental friendliness and high production cost, and achieves easy, active and cheap products, and high cost low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
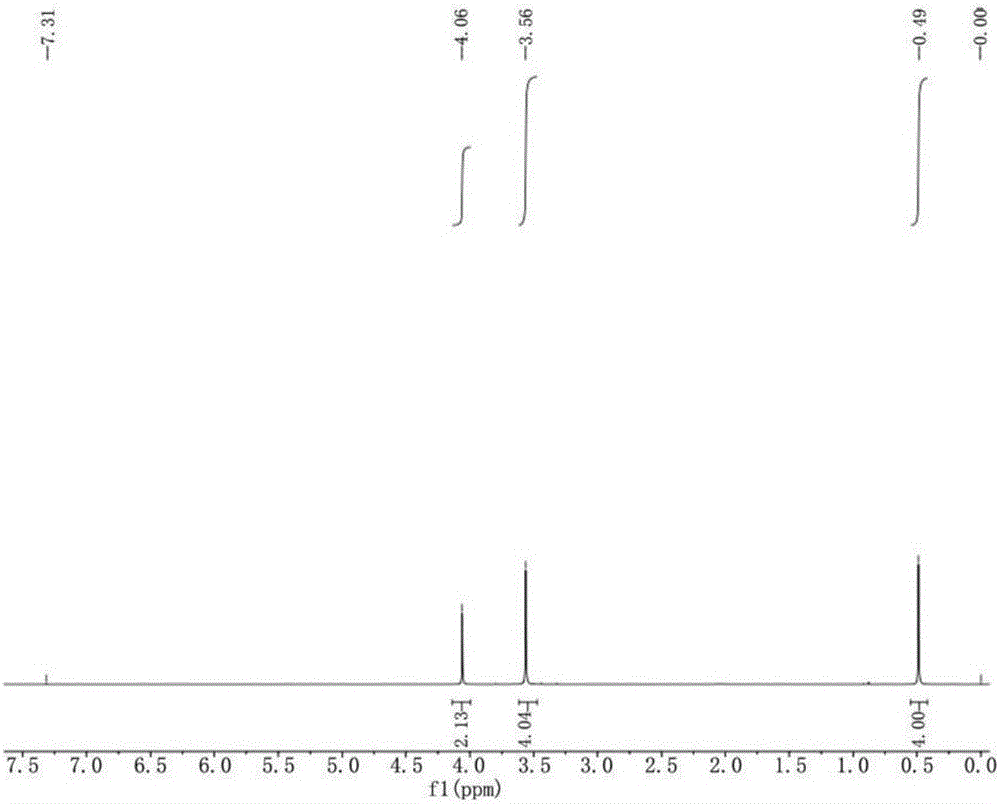
[0038] Example 1: Preparation of 1,1-cyclopropyldimethanol.
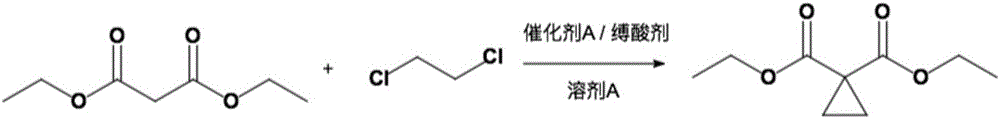
[0039] Add 40g (249.73mmol) of diethyl malonate, 32.14g (324.65mmol) of 1,2-dichloroethane, 3.75g (24.97mmol) of sodium iodide, DMF into a four-necked flask equipped with a mechanical stirring device. 200 mL and 75.93 g (549.41 mmol) of anhydrous potassium carbonate, the reaction system was heated to reflux under stirring conditions, the progress of the reaction was monitored by GC, and the disappearance of diethyl malonate was regarded as the end of the reaction. After the reaction, the reaction solution was cooled to room temperature and filtered. The filter cake was washed with DMF. After combining the filtrate and washing solution, the DMF was evaporated under normal pressure (recoverable and applied) to obtain crude diethyl 1,1-cyclopropyldicarboxylate 42 g (93% yield). The crude product can be used directly in the next reaction without further purification.
[0040] Add 42 g (225.56 mmol) of crude 1,1-cyclopropyl...
Embodiment 2
[0043] Example 2: Preparation of 1,1-cyclopropyldimethanol.
[0044] Into a four-necked flask equipped with a mechanical stirring device was added 40g (249.73mmol) of diethyl malonate, 27.20g (274.70mmol) of 1,2-dichloroethane, 2.07g (12.49mmol) of potassium iodide, 240mL of THF and 52.94 g (499.46 mmol) of anhydrous sodium carbonate, the reaction system was heated to reflux under stirring, the progress of the reaction was monitored by GC, and the disappearance of diethyl malonate was taken as the end of the reaction. After the reaction, the reaction solution was cooled to room temperature and filtered. The filter cake was washed with THF. After the filtrate and the washing solution were combined, the THF was evaporated under normal pressure (recoverable and applied) to obtain crude 1,1-cyclopropyl dicarboxylate 40 g (89% yield). The crude product can be used directly in the next reaction without further purification.
[0045] Into a four-necked flask equipped with a mechanical s...
Embodiment 3
[0046] Example 3: Preparation of 1,1-cyclopropyldimethanol.
[0047] Add 40g (249.73mmol) of diethyl malonate, 37.08g (374.60mmol) of 1,2-dichloroethane, 18.75g (124.87mmol) of sodium iodide, DMA into a four-necked flask equipped with a mechanical stirring device. 300 mL and 62.50 g (624.33 mmol) of anhydrous potassium bicarbonate, the reaction system was heated to reflux under stirring, the progress of the reaction was monitored by GC, and the end of the reaction was the disappearance of diethyl malonate. After the reaction, the reaction solution was cooled to room temperature and filtered. The filter cake was washed with DMA. After the filtrate and washing solution were combined, the DMA was evaporated under normal pressure (recoverable and applied) to obtain crude diethyl 1,1-cyclopropyldicarboxylate 39.5 g (87% yield). The crude product can be used directly in the next reaction without further purification.
[0048] Into a four-necked flask equipped with a mechanical stirring...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com