Preparation method of adenosine cyclophosphate oxide impurity
A technology for cyclic adenosine monophosphate and oxidation of impurities, applied in chemical instruments and methods, preparation of sugar derivatives, organic chemistry, etc., can solve the problems of less research on impurity preparation and higher requirements, and achieve the effect of simple operation and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
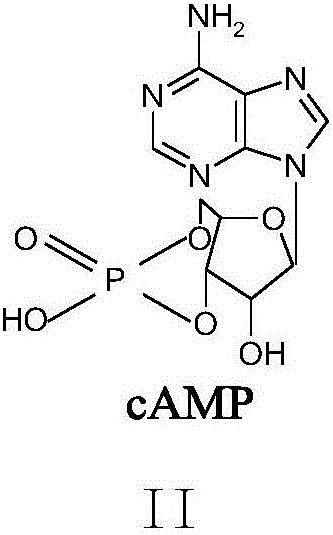
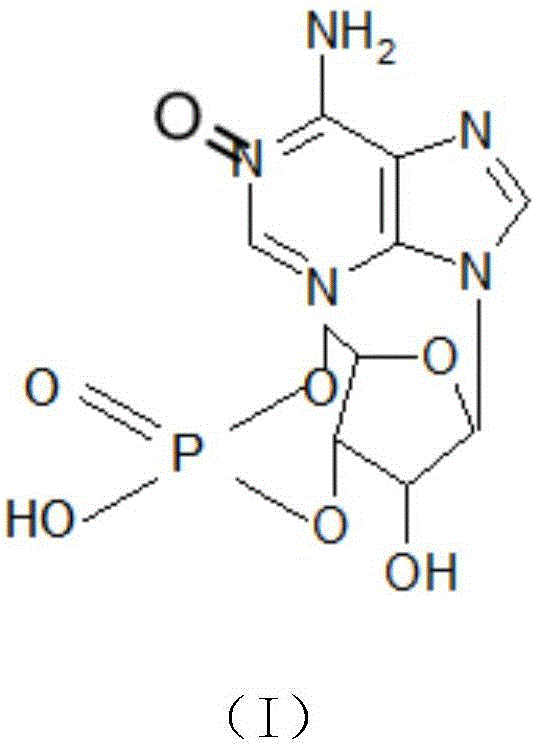
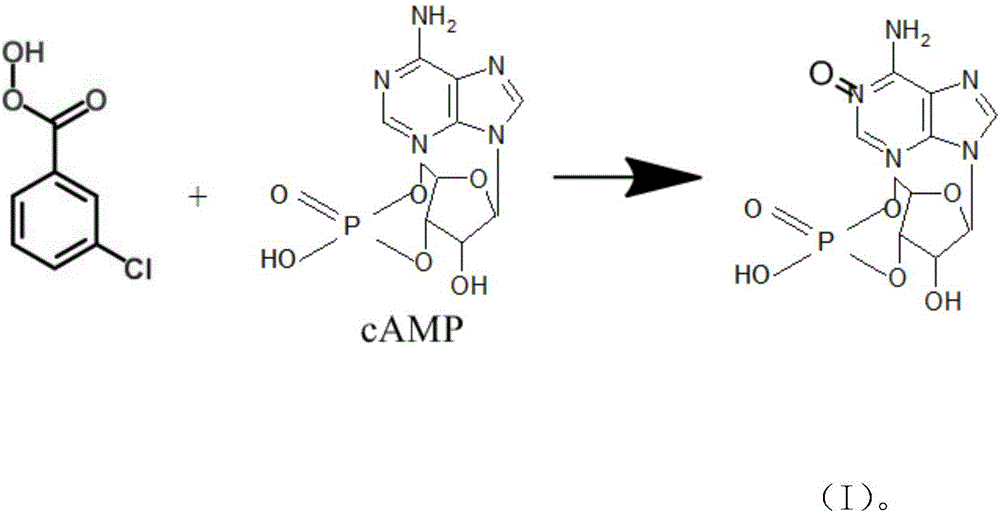
[0022] The preparation method of cyclic adenosine monophosphate oxidized impurities is characterized in that: the preparation method uses cyclic adenosine monophosphate and m-chloroperoxybenzoic acid as starting materials, reacts under the action of solvent and reagent, after reaction The product obtains the cyclic adenosine monophosphate oxidation impurity shown in formula (I) through purification again; Described reaction formula is:
[0023]
[0024] As the present invention, a more specific embodiment is that the solvent is tetrahydrofuran, benzene, methylene chloride, chloroform, carbon tetrachloride, 1,2-dichloroethane, 1,4-dioxane, acetone, butanone, Ethyl acetate, butyl acetate, propyl acetate, acetonitrile, propionitrile, N,N-dimethylformamide, dimethylsulfoxide, acetic acid, propionic acid, preferably N,N-dimethylformamide, di One or more of methyl sulfoxide, acetic acid, and propionic acid.
[0025] The reagent is m-chloroperoxybenzoic acid, hydrogen peroxide ox...
Embodiment
[0028] Cyclic adenosine monophosphate (3.29g, 10mmol) and m-chloroperoxybenzoic acid (2.03g, 10mmol, 85%) were thoroughly mixed in a round bottom bottle, and N,N-dimethylformamide (50ml) was added at room temperature , and the reactants were stirred at 40-50°C for 24 hours. After the reaction solution was stirred at room temperature for 4 days, the reaction solution was washed with water and saturated brine, dried over anhydrous sodium sulfate, and sucked dry to obtain a crude product. Compound A was obtained as a white solid (1.7 g, 5 mmol, 50% yield) by column chromatography. Spectral data:
[0029] 1 H NMR (500MHz,D 2 O)δ11.98(s,1H),7.48(s,1H),7.38(s,1H),4.35(d,1H),4.00(m,2H),3.20(m,1H),2.00-1.87( m,2H);
[0030] MS(ESI):345.05
[0031] Elemental analysis: C: 34.79%, H: 3.50%, N: 20.29%, O: 32.44%, P: 8.97% Spectral data:
[0032] 1 H NMR (500MHz,D 2 O)δ8.58(s,1H),8.35(s,1H),6.99(s,2H),6.16(dd,1H),4.75(m,1H),4.51(m,1H),4.40(m, 1H), 4.28(m, 2H), 3.58(m, 2H);
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com