Method for preparing 2,5-difluorobenzaldehyde through continuous oxidation of 2,5-difluorotoluene
A technology of difluorobenzaldehyde and difluorotoluene, which is applied in the fields of oxidative preparation of carbonyl compounds, chemical instruments and methods, organic chemistry, etc., can solve problems such as increasing production costs and shortening reaction time, and achieves production cost saving and reaction time Short, flexible effects for production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
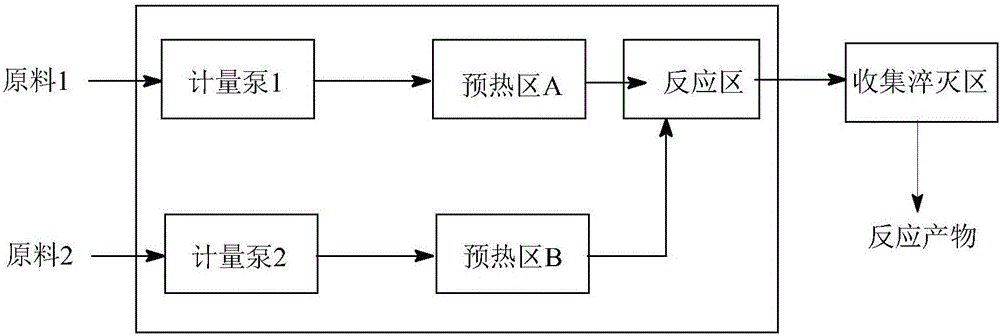
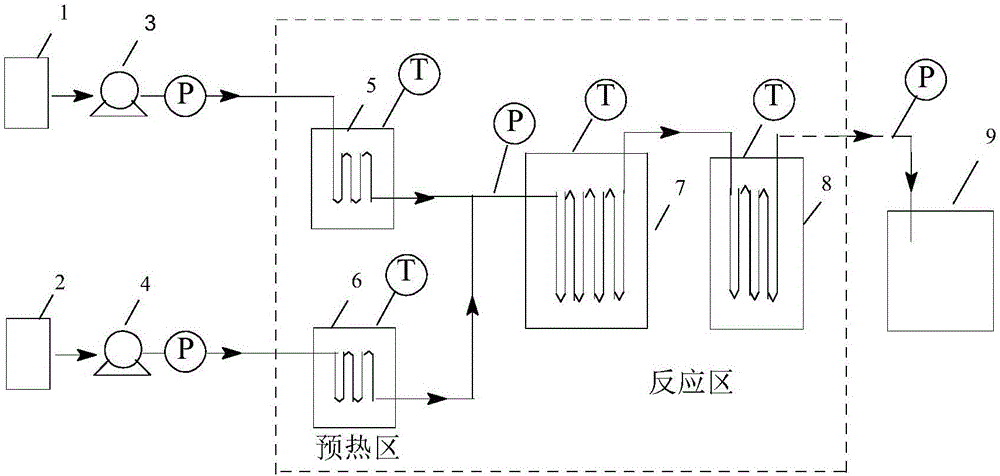
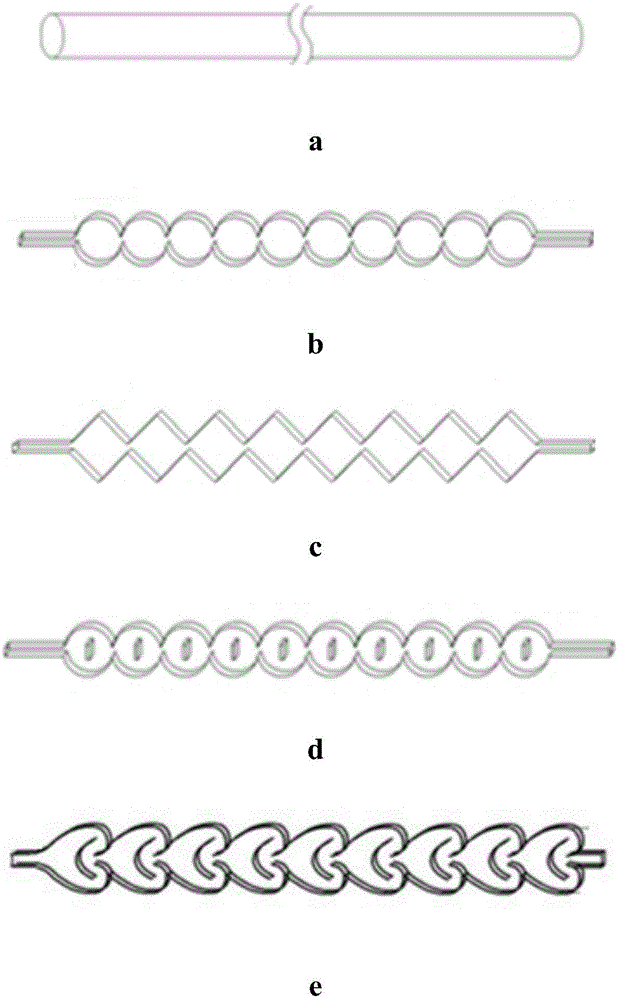
[0030] (1) Device: refer to figure 2 Determine the connection mode of the tubular reactor, the pipeline type is: (3a+3b) direct flow channel + round cake type pulse variable diameter rectangular flat pipeline, the inner diameter and volume of the pipeline are determined according to the flow rate and reaction residence time, and the heat exchange medium is heat conduction Oil.
[0031] (2) Dissolve 1.01g of cobalt acetate and 1.01g of sodium molybdate in 200ml of 2,5-difluorotoluene and 200ml of acetic acid to form a mixed solution. At this time, n(cobalt acetate):n(2,5-difluorotoluene)= 0.0025:1, dissolve 1.01g NaBr in 20% H 2 o 2 form H 2 o 2 -Acetic acid solution, at this time n(sodium bromide):n(2,5-difluorotoluene)=0.0025:1,2,5-difluorotoluene-acetic acid solution and H 2 o 2 -Acetic acid solution is injected in the tubular reactor of continuous heat exchange by the flow rate of 5.33ml / min and 10.67ml / min respectively, at this moment n(H 2 o 2 ): n(2,5-difluoroto...
Embodiment 2
[0033] (1) Device: refer to figure 2Determine the connection mode of the tubular reactor. The pipeline type is: (3a+3c) direct flow channel + oblique square cake pulse variable diameter rectangular flat pipeline. The inner diameter and volume of the pipeline are determined according to the flow rate and reaction residence time. The heat exchange medium is heat transfer oil.
[0034] (2) Dissolve 6.06g of cobalt acetate and 6.06g of sodium molybdate in 200ml of 2,5-difluorotoluene and 200ml of acetic acid respectively to form a mixed solution. At this time, n(cobalt acetate):n(2,5-difluorotoluene)= 0.015:1, 6.06g NaBr dissolved in 20% H 2 o 2 form H 2 o 2 -Acetic acid solution, at this time n(sodium bromide):n(2,5-difluorotoluene)=0.015:1,2,5-difluorotoluene-acetic acid solution and H 2 o 2 -Acetic acid solution is injected in the tubular reactor of continuous heat exchange by the flow velocity of 8.33ml / min and 16.67ml / min respectively, at this moment n(H 2 o 2 ): n(2...
Embodiment 3
[0036] (1) Device: refer to figure 2 Determine the connection mode of the tubular reactor. The pipeline type is: (3a+3d) direct flow channel + enhanced mixed round cake type rectangular flat pipeline. The inner diameter and volume of the pipeline are determined according to the flow rate and reaction residence time, and the heat transfer medium is heat transfer oil. .
[0037] (2) Dissolve 6.06g of cobalt acetate and 6.06g of sodium molybdate in 200ml of 2,5-difluorotoluene and 200ml of acetic acid respectively to form a mixed solution. At this time, n(cobalt acetate):n(2,5-difluorotoluene)= 0.015:1, 6.06g NaBr dissolved in 20% H 2 o 2 form H 2 o 2 -Acetic acid solution, at this time n(sodium bromide):n(2,5-difluorotoluene)=0.015:1,2,5-difluorotoluene-acetic acid solution and H 2 o 2 -Acetic acid solution is injected in the tubular reactor of continuous heat exchange by the flow velocity of 8.33ml / min and 16.67ml / min respectively, at this moment n(H 2 o 2 ): n(2,5-dif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com