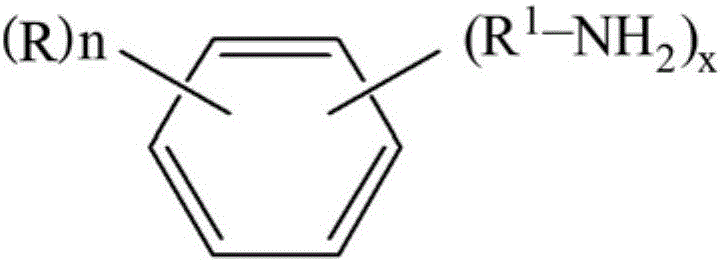
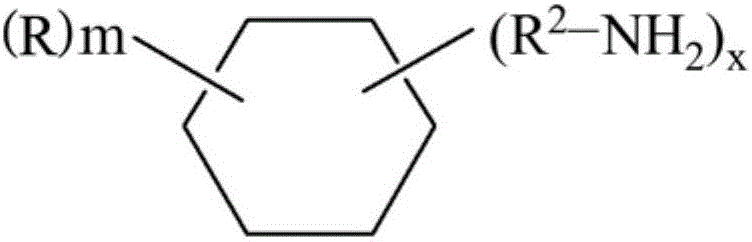
Method for preparing isocyanate by using amine and carbonyl fluoride
A technology using carbonyl fluoride, amine, and isocyanate, which is applied in the field of high-efficiency industrial synthesis, can solve the problems of unavailable raw materials, no recycling process design and recycling effect examples, and low product yield, so as to reduce costs and process complexity , the difficulty of safety supervision and cost reduction, and the effect of reasonable process design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Add 161g (1.0mol) of trifluoromethylaniline (TFMPA) and 600ml of chlorobenzene into a 1.50L stainless steel reactor with a stirrer, fill it with 165g (2.5mol) of carbonyl fluoride, and then close the system, react at room temperature for 2 hours, Then use the reactor as the rectifying tower tower tank, direct rectification, adjust the tower tank temperature, ensure that the system pressure is 1Mpa, the tower top temperature is-40 ℃, and the tower top takes out carbonyl fluoride 90g, and the recovery rate is 91%, then Raise the temperature of the tower kettle to about 130°C, ensure that the system pressure is 0.5Mpa, the tower top temperature is 20°C, and 18g of hydrogen fluoride is produced from the tower top, with a recovery rate of 90%. Then react at normal pressure and temperature of 130°C for 2 hours to remove hydrogen fluoride. After the reaction, the temperature of the refrigerant at the top of the tower is 20°C, and 19g of hydrogen fluoride is produced at the top ...
Embodiment 1A
[0062] The only difference from Example 1 is that the amount of carbonyl fluoride added is 185g (2.8mol). The obtained trifluoromethylphenyl isocyanate (TFMPI) crude product was 174.8g, the purity was 86.0%, and the product yield was 80.4%.
Embodiment 1B
[0064] The only difference from Example 1 is that the amount of carbonyl fluoride added is 264g (4mol). The obtained trifluoromethylphenyl isocyanate (TFMPI) crude product was 201.9 g, the purity was 74.0%, and the product yield was 79.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com