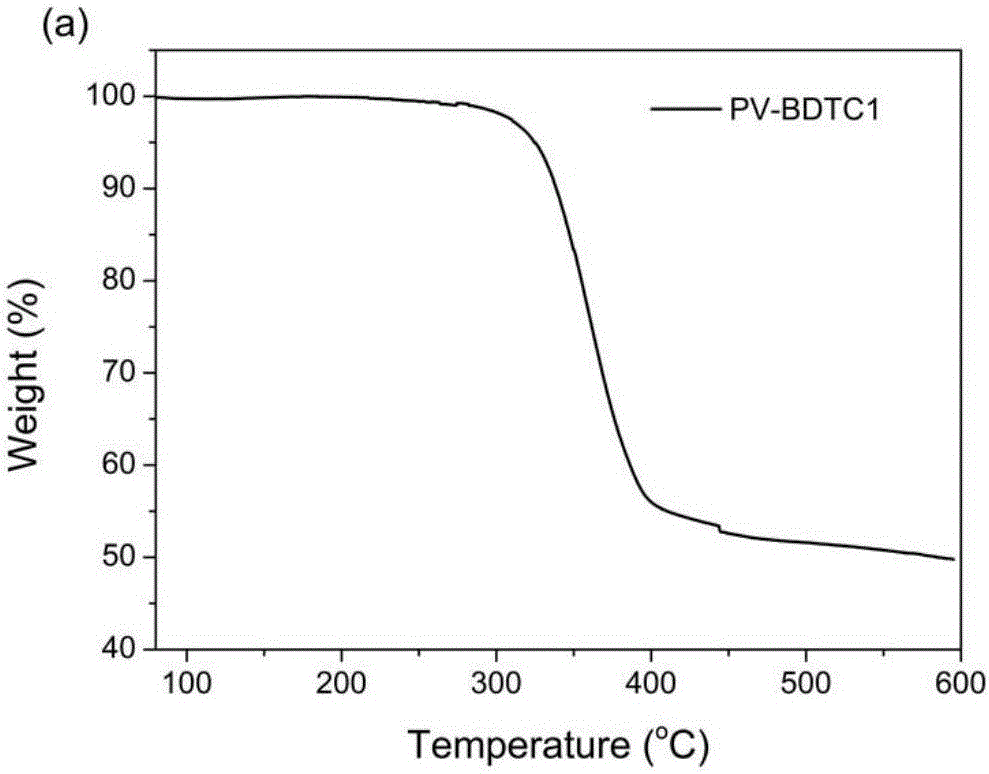
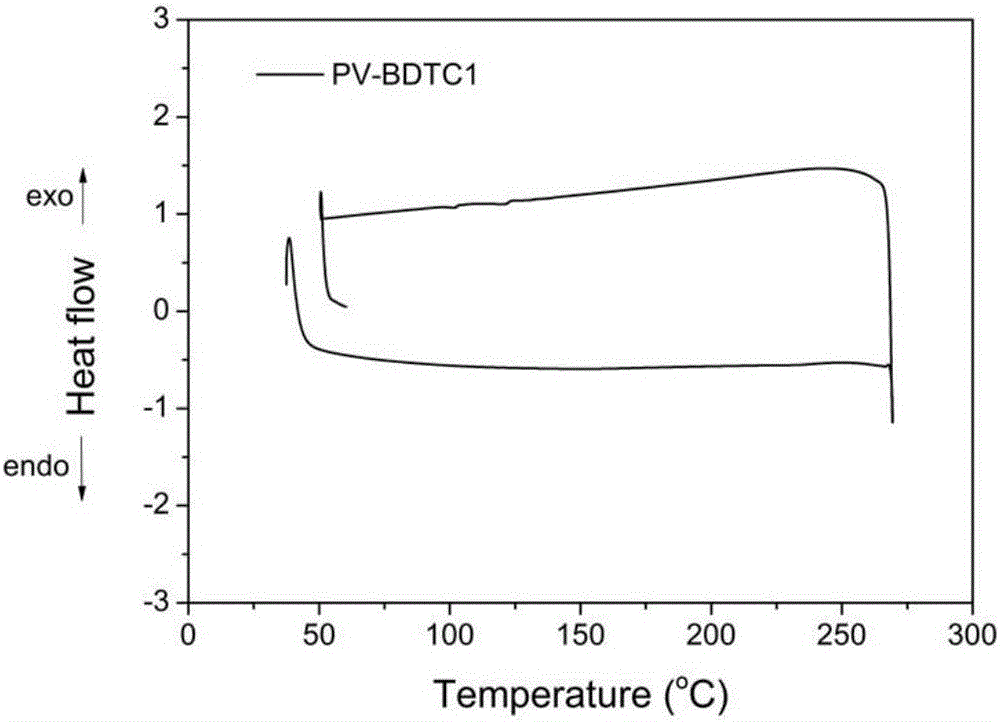
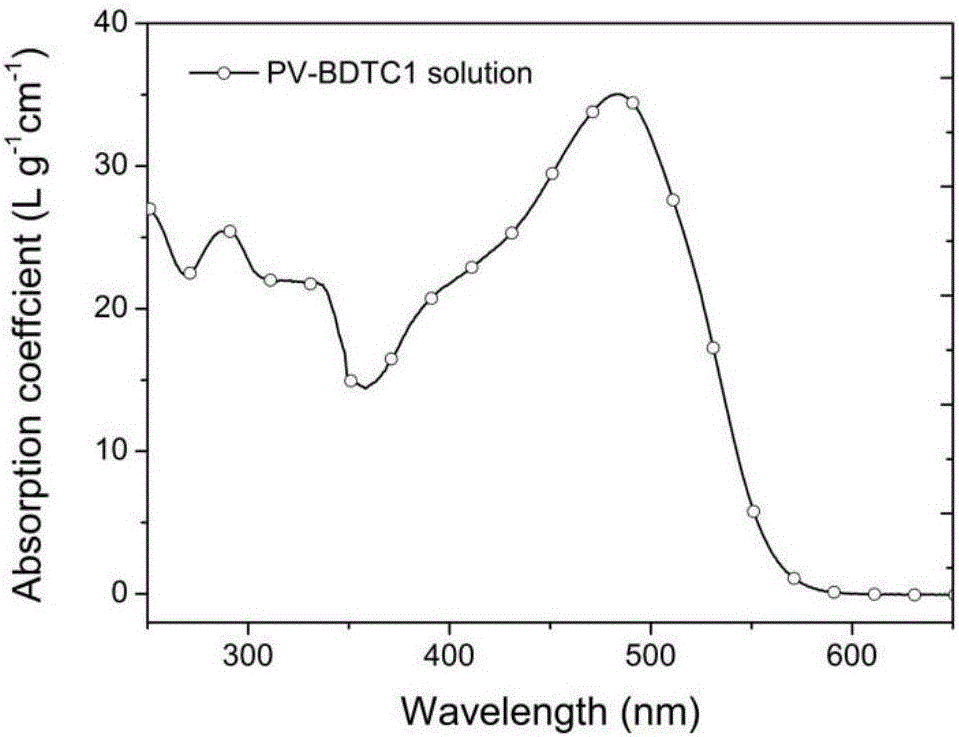
D-A type broad-band gap polymer photovoltaic materials based on benzodithiophene-2,6-diformate and application of D-A type broad-band gap polymer photovoltaic materials
A technology of benzodithiophene and dicarboxylate, applied in photovoltaic power generation, electrical components, circuits, etc., can solve the problems of low energy conversion efficiency and few varieties of wide-bandgap photovoltaic materials, achieve large open circuit voltage, increase electron acceptance ability, efficient energy conversion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Synthesis of 4,8-dibromobenzo[1,2-b:4,5-b']dithiophene-2,6-di[(2-butyl)octyl carboxylate]
[0071]
[0072] 1.1 Synthesis of 2,5-dibromobenzene-1,4-dicarbaldehyde
[0073] In a 100mL single-necked bottle, dissolve 2,5-dibromo-1,4-dimethylbenzene (3.9g, 15mmol) in a mixed solution of 20mL acetic acid and 40mL acetic anhydride, stir magnetically, and slowly add dropwise at 0°C After concentrated sulfuric acid (14mL), add CrO2 in batches 3 (6.0g), reacted at 0°C for 3h. After the reaction was completed, the reaction solution was poured into 100 mL of ice water, and a large amount of white solid was precipitated, filtered with suction, and washed with water. The solid was transferred to a 100mL single-necked bottle, 20mL of water, 20mL of ethanol and 2mL of concentrated sulfuric acid were added, and heated to reflux for 12h. Cool naturally, filter with suction, and wash the solid with water and methanol alternately for several times. Drying in vacuo gave a pale yello...
Embodiment 2
[0081] 4,8-Bis-(2-(5-bromothienyl)benzo[1,2-b:4,5-b']dithiophene-2,6-bis[(2-butyl)octyl carboxylate ](M1) synthesis
[0082]
[0083] 2.1 Synthesis of 4,8-bis-(2-thienyl)benzo[1,2-b:4,5-b']dithiophene-2,6-bis[(2-butyl)octyl formate]
[0084] In a 100mL single-necked bottle, sequentially add (4,8-dibromobenzo[1,2-b:4,5-b']dithiophene-2,6-bis[(2-butyl)octyl carboxylate] (0.77g, 1.0mmol), tetrakis(triphenylphosphine)palladium (60mg), 2-tributyltinthiophene (1.12g, 3.0mmol), 30mL toluene, system under nitrogen protection. Stir the reaction at 100°C for 18h at temperature control. Stop the reaction , After the reactant was cooled to room temperature, it was poured into 100mL distilled water and extracted with dichloromethane (3 × 30mL).The combined organic phase was dried with anhydrous magnesium sulfate, filtered, and the filtrate was distilled under reduced pressure to remove the solvent, and the residue Using petroleum ether:dichloromethane mixed solution (v / v, 2:1) as elue...
Embodiment 3
[0088]
[0089] Synthesis of Polymer PV-BDTC1
[0090] In a 25mL two-necked flask, add M1 (154mg, 0.2mmol), trimethyltinbenzodithiophene (187mg, 0.2mmol), tris(dibenzylideneacetone) dipalladium (3mg), tris(o-tolyl) ) phosphorus (6mg), oxygen-free toluene 6mL. Under the protection of nitrogen flow, the temperature was controlled at 110° C. for 24 h. Cool naturally, add 10mL of toluene to dilute the reaction solution, add dropwise to 100mL of methanol to settle. After suction filtration, the solid polymer was subjected to Soxhlet extraction with methanol, ether, and chloroform in sequence, and the chloroform extract was concentrated, dropped into methanol to settle. The solid was collected by suction filtration and dried in vacuum. 200 mg of red solid was obtained with a yield of 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com