A kind of preparation method of hydrocracking catalyst
A hydrocracking and catalyst technology, which is applied in the direction of physical/chemical process catalysts, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problem that the active components of the catalyst cannot function efficiently, affect the efficient performance of the acidic function, and the coking failure of the catalyst. Improve the selectivity and nitrogen resistance of medium oil, improve the ability to resist nitrogen poisoning, and improve the utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
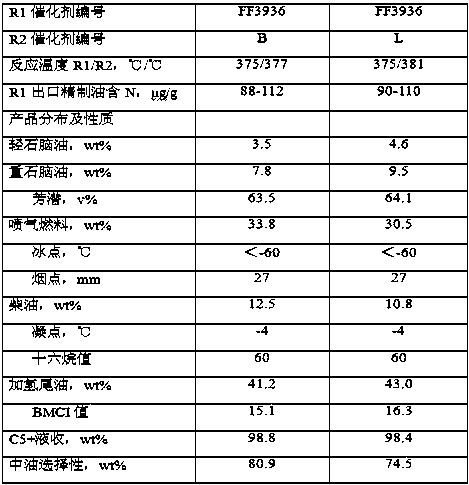
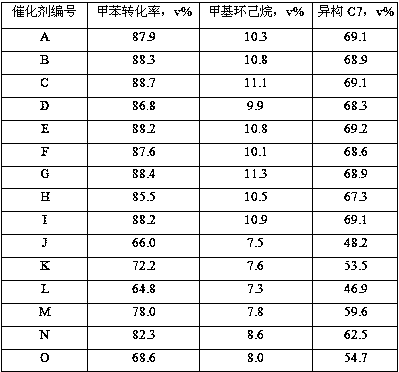
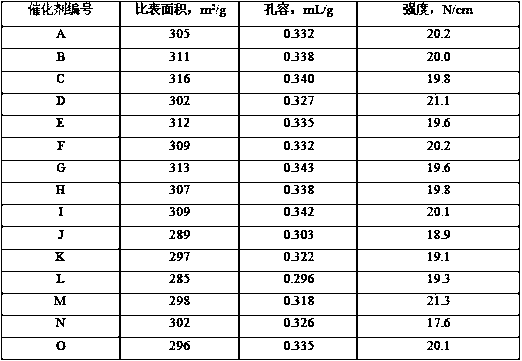
Image
Examples
preparation example Construction
[0034] The preparation method of the hydrocracking catalyst provided by the present invention specifically comprises:
[0035] (1) Add alkaline solution B containing tungsten to acidic solution A containing magnesium, aluminum and silicon until the pH is 10-12 to obtain a mixed slurry;
[0036] Prepare magnesium, aluminum, and silicon-containing acidic solution A according to the content ratio of the catalyst components. The aluminum-containing salt can be one or more of aluminum chloride, aluminum nitrate, aluminum sulfate, etc., and the magnesium-containing salt can be magnesium chloride, nitric acid, etc. One or more of soluble magnesium salts such as magnesium and magnesium sulfate, the silicon source can be one or more of water glass, silica sol, etc.; the acidity can be one or more of inorganic or organic acids such as hydrochloric acid, nitric acid, and acetic acid One or more adjustments, it is advisable that the acidic salt solution is not turbid;
[0037] The auxili...
Embodiment 1
[0053] (1) Add 2L of clean water to a gelling tank and raise the temperature to 50°C;
[0054] Add 1.5L of clean water, 30mL of concentrated hydrochloric acid, 970g of aluminum chloride, 50g of magnesium chloride into a container, stir to dissolve, drop in the 2 1L of 131g / L dilute water glass solution, stir evenly, and prepare acidic solution A;
[0055] Prepare 2.2L of sodium hydroxide solution with a concentration of 15wt% in a container, add 46g of sodium tungstate, stir to dissolve, and prepare alkaline solution B;
[0056] Fix the flow rate of the acidic solution A, adjust the flow rate of the alkaline solution B, and flow into the gelation tank, control the gelation temperature at 50°C, the pH of the slurry at 10.8±0.2, and complete the gelation within 30 minutes. When the reaction is over, the A solution and B solution must all be added to the gel tank, and stirred for 10 minutes after the gel is completed;
[0057] (2) Add 2L of clean water to a gelling tank and rai...
Embodiment 2
[0066] Take a part of the shaped bar C3 with a dry basis content of 65wt% prepared in Example 1, spray and soak it with 40mL of an organic acid mixed aqueous solution containing 1.5g of acetic acid and 3.5g of citric acid, then put it into a glass container, and place it in a constant temperature water bath at 50°C. Insulated for 40 minutes; drying and roasting conditions were the same as those in Example 1 to obtain catalyst B of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com