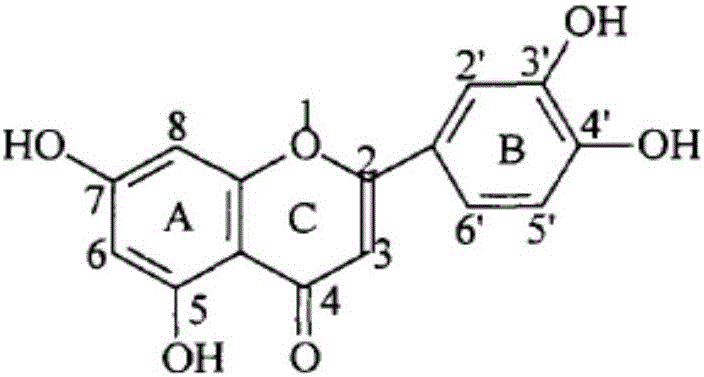
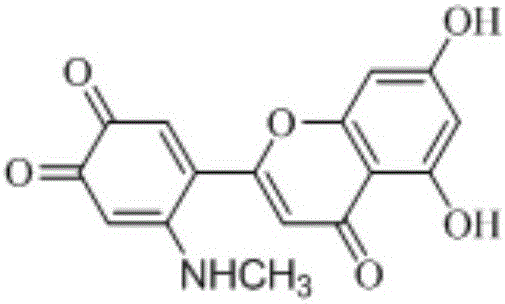
Preparation method of 6'-amino derivatives based on luteolin structure and application thereof
A technology of amino derivatives and luteolin, which is applied in the field of preparation of luteolin derivatives, achieves the effects of mild conditions, enhanced drug efficacy, product separation and purification, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of luteolin and fatty amine
[0035] Weigh 0.1430 (0.5 mmol) luteolin, pour it into 50 mL of 95% ethanol, and dissolve it under the action of ultrasonic waves. Weigh 1.0 mL (0.1035 g, 0.69 mmol) of 30% methylamine in ethanol, dissolve it in 10 mL of ethanol, and drop the solution into the ethanol solution of luteolin. Add pH = 9.3 carbonate buffer solution dropwise, and measure the final pH of the reaction mixed solution with precision pH test paper to be 9.2, and the mixed solution quickly turns dark red. A reflux condensing device was set up, and the reaction was carried out at 30°C. At the same time, the progress of the reaction was tracked by TLC (developing agent ethanol: ethyl acetate volume ratio = 1.5:3, adding a small amount of ammonia water), the reaction solution was slowly cooled to room temperature, and then extracted three times with 20 mL / ethyl acetate to obtain a bright yellow extract. liquid; the mother liquor was extracted t...
Embodiment 2
[0041] Weigh 0.1430 g (0.5 mmol) of luteolin, add it into 50 mL of 95% ethanol, and dissolve it under the action of ultrasonic waves. Weigh 0.0683 g (0.5 mmol) of an aqueous solution of 33% dimethylamine and dissolve it in 10 mL of ethanol solution, and drop the solution into the ethanol solution of luteolin. Add pH = 9.3 carbonate buffer solution dropwise, and measure the final pH of the reaction mixed solution with precision pH test paper to be 9.2, and the mixed solution quickly turns dark red. A reflux condensing device was added, and the reaction was carried out at 50°C for 24h. At the same time, the progress of the reaction was tracked by TLC (ethanol: ethyl acetate volume ratio = 1:5), the reaction solution was slowly cooled to room temperature, and then extracted three times with 10 mL / ethyl acetate to obtain a yellow extract (i); The mother liquor was extracted four times with 10 mL / time of n-butanol to obtain a reddish-brown extract (ii) and an extract water raffina...
Embodiment 3
[0047] Weigh 1.00 g (0.0035 mol) of luteolin and add 100 mL of absolute ethanol to obtain a pale yellow luteolin suspension. Weigh 0.29 g (0.0105 mol) of ethylamine, dissolve it in a small amount of ethanol solution, and pour it into the luteolin solution. Then weigh 6.90g of sodium hydroxide, add 100mL of water to prepare a sodium hydroxide solution, slowly add it dropwise to the reaction solution, use precision pH test paper to measure the final pH of the reaction mixture solution to be 9.2, and reflux at 50°C. A small amount of ammonia water was added as a developing solvent with methanol: chloroform volume ratio = 1.5:9.0, and plate spotting was carried out during the experimental reaction to track the reaction progress. After the reaction was completed, the reaction solution was slowly cooled to room temperature, filtered with suction, and the filter residue was collected. The mother liquor was rotary evaporated, filtered, and the filter residue was mixed with the filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com