Self-emulsifying solid preparation containing ivermectin drug
A solid preparation, ivermectin technology, applied in the field of preparation of self-emulsifying solid dispersion containing ivermectin drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
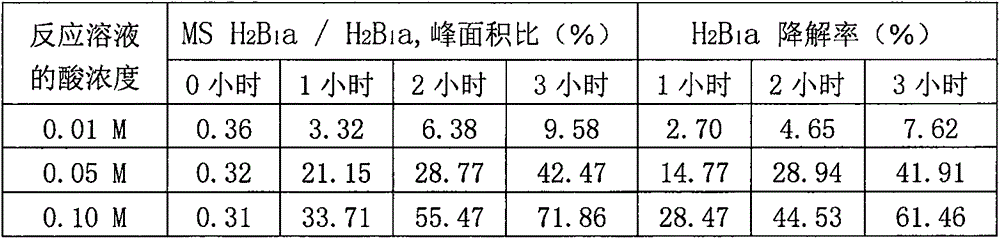
[0040] Example 1. Screening of surfactants by acid-catalyzed degradation test of microemulsions containing different surfactants
[0041] (1) Microemulsion composition: The surfactants used in the preparation of microemulsions include the surfactants described in Table 5 and polyoxyethylene castor oil condensate, polyethylene glycol (40) palm kernel oil, polyethylene glycol (60) ) Corn oil, polyethylene glycol (60) corn oil glycerides, polyethylene glycol (60) almond oil, polyethylene glycol (50) castor oil, the effective component is ivermectin (0.6%), micro The oil phase in the emulsion is ethyl acetate, the co-emulsifier is 1,2-propylene glycol, and water is added to 100% of the volume of the microemulsion. (2) Acid-catalyzed degradation test method: Take 1ml of microemulsion, add 19ml of 0.1M hydrochloric acid solution, react at 36-37℃ for 1 hour, adjust the pH with alkali and filter, and detect MS H in the filtrate by HPLC 2 B 1 a and H 2 B 1 a, record the chromatogram, calc...
Embodiment 2
[0042] Example 2. Screening of surfactants through the acid catalytic degradation test of ivermectin
[0043] (1) The basic formula of the preparation (weight ratio): 0.6% ivermectin, 10% surfactant, 8% glyceryl monostearate, 0.8% 1,2-propanediol, between 40-100 mesh The corncob flour is added to 100%.
[0044] (2) Preparation method of the preparation: mix ivermectin, surfactant, glyceryl monostearate, 1,2-propanediol, stir and dissolve at 80-85°C, add corn cob powder, fully Stir and mix well and cool down to room temperature to get.
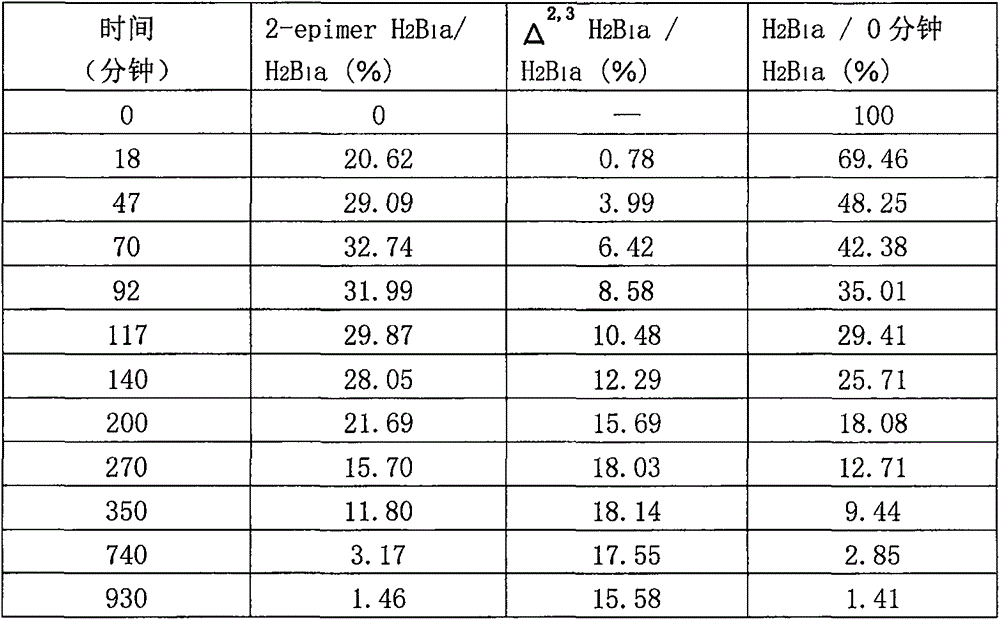
[0045] (3) Acid catalytic degradation test and test results
[0046] Take 1 gram of sample into a 25 ml test tube with stopper, add 18 ml of water, shake for 5 minutes, then add 2 ml of 1M hydrochloric acid solution, mix well, incubate at 36-37°C for 1 hour, draw 5 ml of reaction solution, add 10% About 0.24 ml of sodium hydroxide solution, mix well, add methanol to make the volume up to 10 ml, mix well, filter with 0.45um membrane, detect the filtra...
Embodiment 3
[0050] Example 3. Preparations containing HEL-40 and different oily media and their acid-catalyzed hydrolysis test
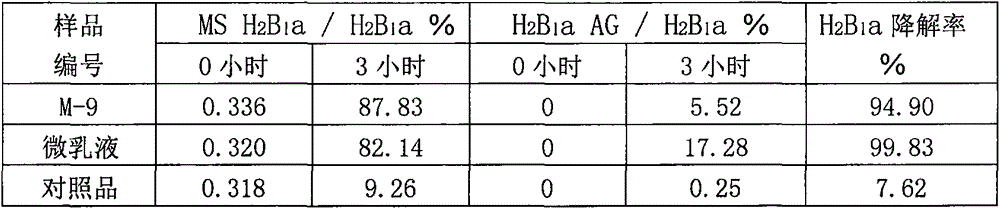
[0051] (1) The basic formulation (weight ratio) of the preparation: 0.6% ivermectin, HEL-4010%, 1,2-propanediol 1%, an appropriate amount of oily medium (see Table 6), and corncob powder to 100%. (2) Acid-catalyzed hydrolysis test: Take a sample of 1.00 g in a 25 ml test tube with a stopper, add 18 ml of water, shake for 5 minutes, then add 2 ml of 1M hydrochloric acid solution, mix well, incubate at 36-37°C for 2 hours, and absorb 5ml of reaction solution, add about 0.24ml of 10% sodium hydroxide solution, mix well, add methanol to make the volume to 10ml, mix well, filter with 0.45um membrane, and detect the filtrate by HPLC (inject 20ul), record the peak Area value, calculate MS H 2 B 1 a peak area value and H 2 B 1 a Ratio of peak area value (%). The test results are shown in Table 6.
[0052] Table 6. The effect of oily medium on the acid-catalyzed hydrolysis ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com