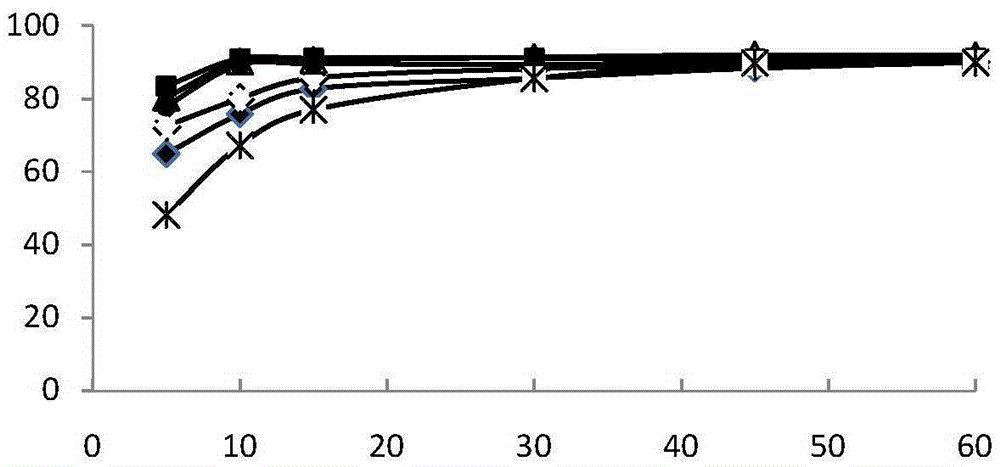
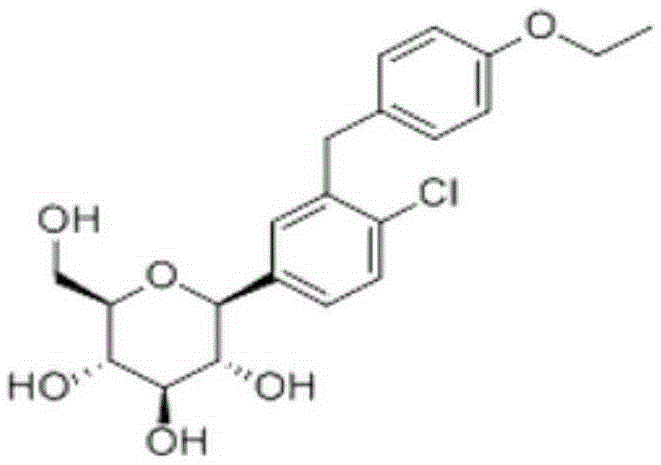
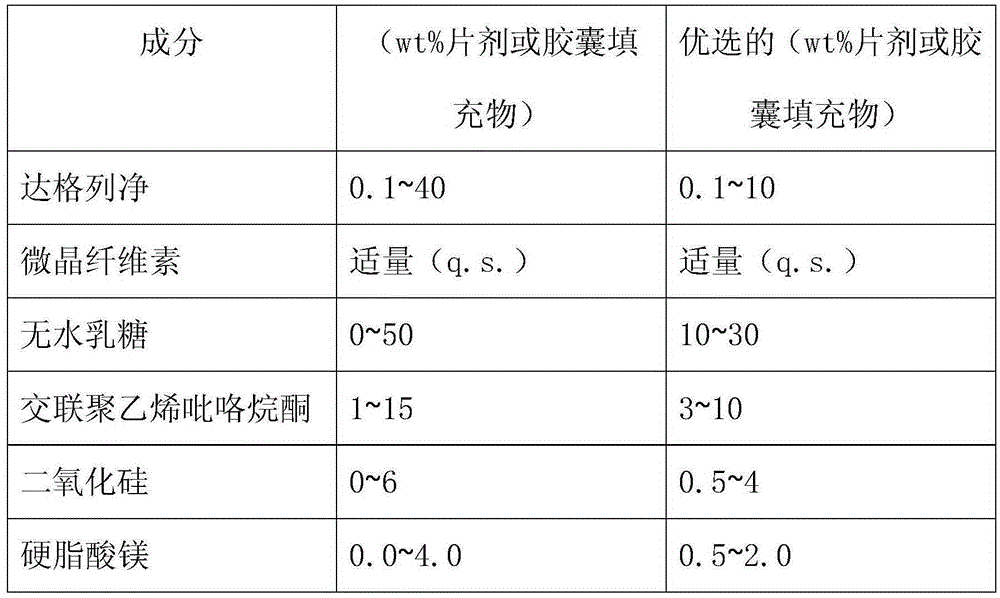
Dapagliflozin medicinal composition and preparation method thereof
A composition and drug technology, applied in the direction of drug combination, pharmaceutical formula, sugar-coated pills, etc., can solve the problems of increasing production safety hazards, cumbersome procedures, reducing particle size, etc., to save costs and production time, simple preparation process, The effect of increasing the dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 9
[0052] The particle size of the dapagliflozin raw material used in Example 9 is D90=33 μm, which is obtained by pulverizing a commercial product (D90=110 μm) by jet milling. Jet mill model: AO (Yixing Qingxin Powder Machinery Company), operating conditions: pump pressure 0.4MPa.
Embodiment 10
[0053] The particle size D90 of the dapagliflozin raw material used in Example 10 was 110 μm. For commercial products.
Embodiment 1
[0054] Embodiment 1: Preparation of dapagliflozin cellulose composition granules:
[0055] Weigh 10g of dapagliflozin and 40g of microcrystalline cellulose, mix them and add them into a hot-melt extruder at a temperature of 70°C. After extrusion, take them out and cool them, and use a mechanical pulverizer to pulverize them. Pass the pulverized materials over 30 mesh sieve to obtain 40 g of dapagliflozin cellulose composition granules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com