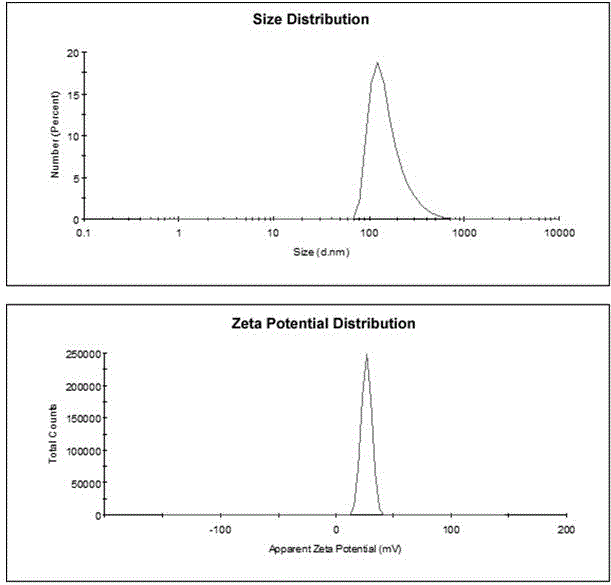
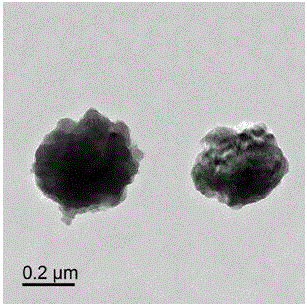
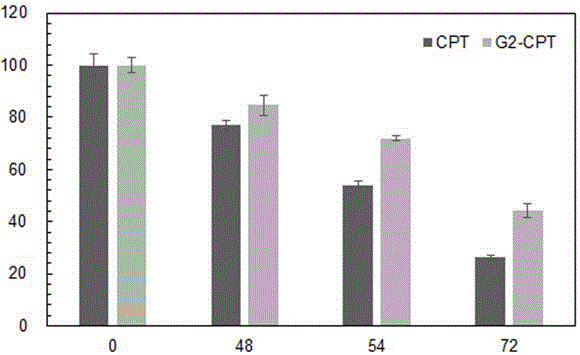
Delivery system capable of realizing co-loading gene and drug, preparation method of delivery system, and application
A gene and anti-tumor drug technology, applied in the field of nano-pharmaceutical preparations, can solve the problem of not considering the influence of the treatment effect, and achieve the effect of improving the encapsulation rate and drug loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] (1) Use divergence method or convergence method or divergence-convergence method to synthesize more than two generations of dendrimers (refer to the method described in the patent literature with publication number CN 103554923A), or use commercially available dendrimers molecular;
[0062] (2) Protect the peripheral functional groups of the resulting dendrimers containing amino groups and / or guanidine groups at the periphery, expose only one or two functional groups at one end, and then connect one end of the dendrimers through the exposed functional groups branch reduction sensitive keys;
[0063] (3) The other end exposed to the reduction-sensitive bond is grafted with a hydrophobic antineoplastic drug or its prodrug;
[0064] (4) Remove the amino and / or guanidine protecting groups on the periphery of the dendrimer to obtain a dual-response lipid prodrug.
[0065] As an alternative, in the above step (3), a hydrophobic antitumor drug with a suitable active group ca...
Embodiment 2
[0068] (1) Dissolve the plasmid DNA or RNA in sterile DEPC water (0.1% diethylpyrocarbonate) to prepare a gene solution of appropriate concentration; or mix the lipid prodrug prepared in Example 1 and the commercially available lipid Proteolytic molecules (DOPE, DSPE, cholesterol, etc.) or amphiphilic molecules that replace the hydrophobic groups of lipid prodrugs with alkane chains are mixed at a ratio of 1:0~1:1 and added to HBG buffer solution by solution dispersion method to prepare 0.1~10 mg / mL solution A.
[0069] (2) Mix the solution A obtained in the above steps with the gene solution, and incubate at room temperature for 20 minutes to obtain a cotransport drug delivery system.
[0070] Optionally, the mass ratio of the lipid prodrug to the gene is 0.1:1-50:1.
[0071] As an option, other drugs or magnetic nanoparticles can also be added in the above step (2) to prepare ternary or quaternary compounds.
[0072] Experiments have shown that the obtained co-transport dr...
Embodiment 3
[0073] Example 3: Preparation of second-generation arginine-cystamine-succinic anhydride-camptothecin (Arg(G2)-SS-SUC-CPT)
[0074] Take 1 mmol of camptothecin (CPT) and 3 mmol of succinic anhydride (SUC) at 0°C under nitrogen protection, add dichloromethane solvent, slowly drop 3 mmol of condensing agent (1,8-diazabicyclo [5.4.0] Undec-7-ene (DBU)); then react at room temperature for 4 hours, after the reaction, add water to terminate the reaction. The resulting solution was precipitated with 1% HCl solution. The precipitate was washed successively with 1% HCl solution and water to obtain succinic anhydride-camptothecin.
[0075] Take succinic anhydride-camptothecin, monoprotected cystamine, condensation agent (such as: 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride EDC, catalyst 1-hydroxybenzene Triazole (HOBt), base (N, N-diisopropylethylamine DIPEA) according to the molar ratio of 1: 1: 2: 2: 4, at 0 ° C, under nitrogen protection conditions, add dichlorometh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com