Method for comprehensive treatment and utilization of high-zinc-content waste acid
A comprehensive treatment, hydrochloric acid solution technology, applied in the direction of zinc halide, zinc oxide/zinc hydroxide, iron halide, etc., can solve the problems of complex process, high cost, and no consideration of the investment cost of the final recovery of ammonia corrosion-resistant equipment, etc., to achieve The effect of simple process and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
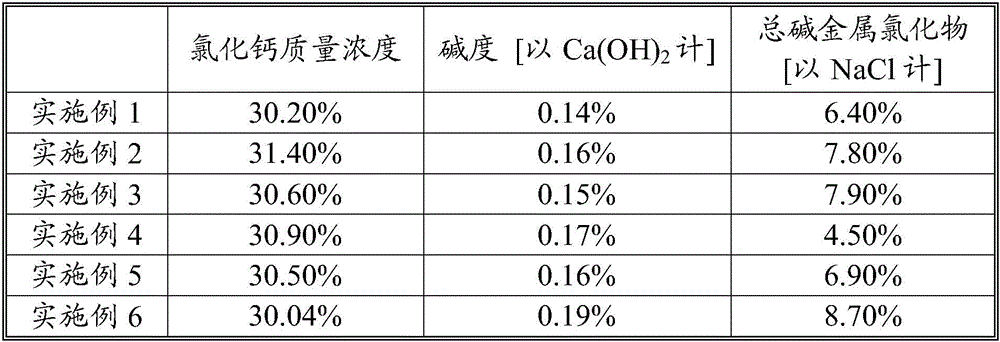
Examples
Embodiment 1
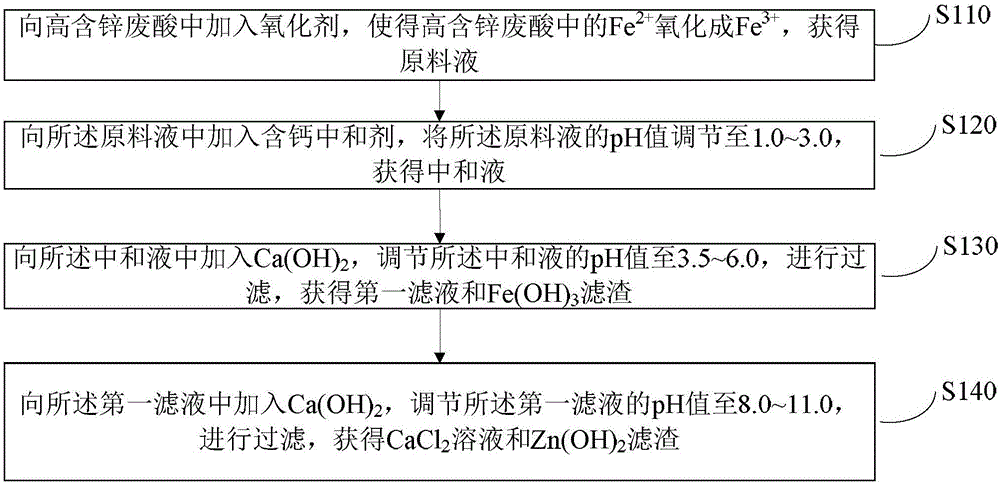
[0055] 2.3t high zinc-containing waste acid (which contains 3.14% iron, 12.89% zinc, and acidity [calculated as HCl] is 3.87%) is subjected to pressure filtration, and the filtrate is poured into 1# reaction tank, and sodium chlorate is used as an oxidant. Add sodium chlorate to the 1# reaction tank for oxidation reaction. After the oxidation reaction is over, it is detected that there is no Fe in the solution. 2+ , it is considered qualified for oxidation.
[0056] Then add calcium carbonate to carry out the acid consumption reaction, adjust the pH value to 1.0 and then finish the acid consumption, then add calcium hydroxide to carry out the iron precipitation reaction, adjust the pH value to 3.5 and then finish the iron precipitation reaction, start the discharge pump, and send the feed liquid to Press filter to the filter press, and the filter residue is sent to FeCl 3 The iron pool in the preparation workshop is used as raw material for the production of ferric chloride w...
Embodiment 2
[0059] 2.12t of high zinc-containing waste acid (which contains 3.14% iron, 12.89% zinc, and 3.87% acidity [calculated as HCl]) is subjected to pressure filtration, and the filtrate is poured into 1# reaction tank, and sodium chlorate is used as an oxidant. Add sodium chlorate to the 1# reaction tank for oxidation reaction. After the oxidation reaction is over, it is detected that there is no Fe in the solution. 2+ , it is considered qualified for oxidation. Then add calcium carbonate to carry out the acid consumption reaction, adjust the pH value to 1.5 and then finish the acid consumption, then add calcium hydroxide to carry out the iron precipitation reaction, adjust the pH value to 4.5 and then finish the iron precipitation reaction, start the discharge pump, and send the feed liquid to Press filter to the filter press, and the filter residue is sent to FeCl 3 The iron pool in the preparation workshop is used as raw material for the production of ferric chloride water pur...
Embodiment 3
[0062] 1.86t high zinc-containing waste acid (which contains 9.14% iron, 5.82% zinc, and acidity [calculated as HCl] is 3.13%) is subjected to pressure filtration, and the filtrate is poured into 1# reaction tank, and sodium chlorate is used as an oxidant. Add sodium chlorate to the 1# reaction tank for oxidation reaction. After the oxidation reaction is over, it is detected that there is no Fe in the solution. 2+ , it is considered qualified for oxidation. Then add calcium carbonate to carry out the acid consumption reaction, adjust the pH value to 2.8 and then finish the acid consumption, then add calcium hydroxide to carry out the iron precipitation reaction, adjust the pH value to 5.5 and then finish the iron precipitation reaction, start the discharge pump, and send the feed liquid to Press filter to the filter press, and the filter residue is sent to FeCl 3 The iron pool in the preparation workshop is used as raw material for the production of ferric chloride water puri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com