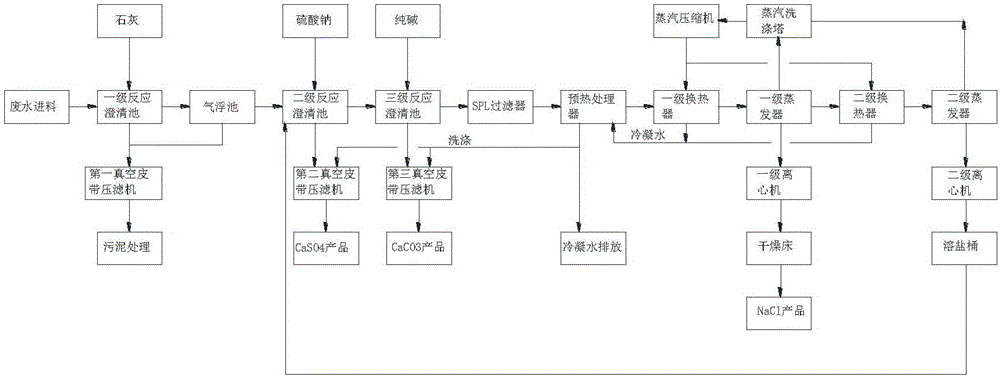
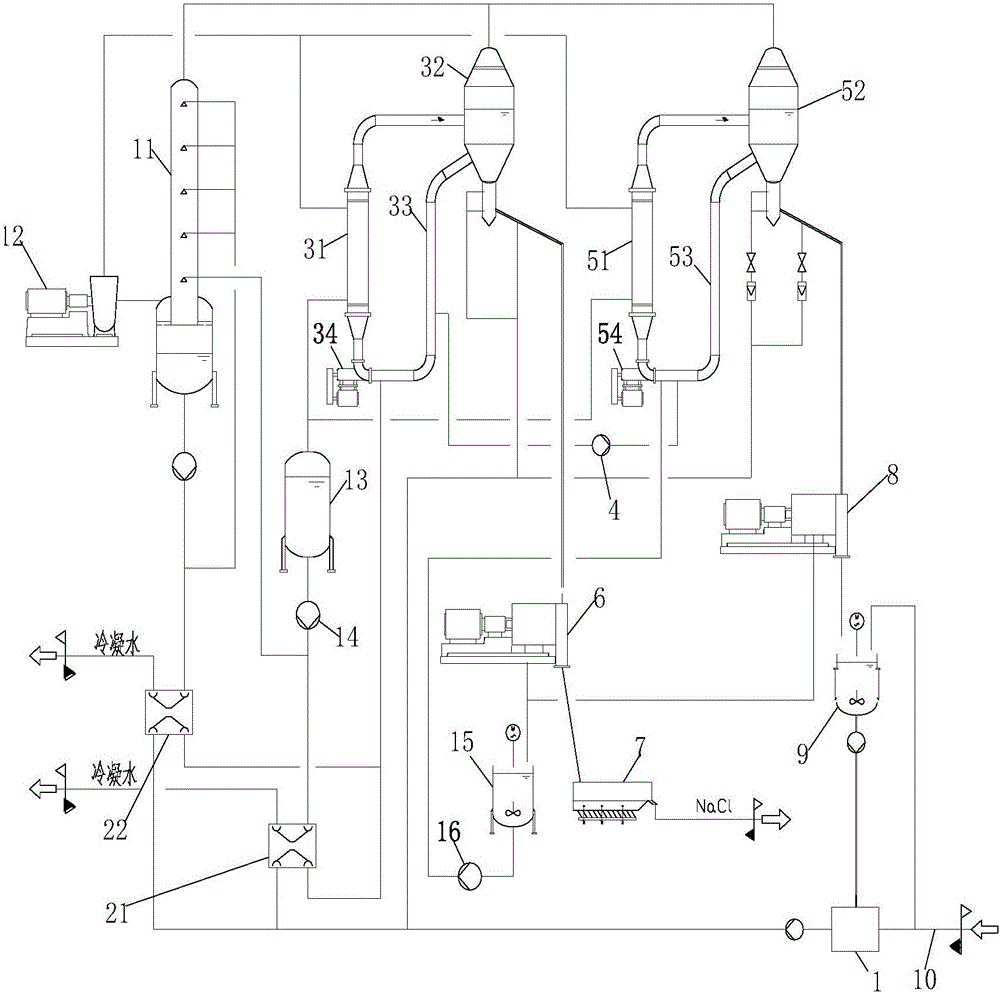
Zero-emission process and system for desulfurization wastewater
A desulfurization wastewater and zero-discharge technology, applied in multi-stage water treatment, water/sewage treatment, heating water/sewage treatment, etc., can solve problems such as pollution, large environmental pollution, and no economic value of calcium salts and magnesium salts. The effect of reducing the amount of production, increasing the variety, and increasing the value of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Evaporation at 50°C
[0072] Solubility components of sodium chloride-sodium sulfate-water, as shown in Table 3:
[0073] Table 3 Solubility of sodium chloride-sodium sulfate-water at 50℃
[0074]
[0075]
[0076] According to the mutual solubility relationship of sodium chloride-sodium sulfate at 50°C, the first-stage evaporation should control the sodium sulfate component to be less than 5.3%, and the sodium chloride component is greater than 24.2%, so that sodium chloride crystals can be precipitated, and Sodium sulfate did not precipitate out. That is, the primary evaporation is:
[0077] M(Na 2 SO 4 ) / (M total-M evaporation)*100%<5.3%;
[0078] M evaporation 2 SO 4 )*100% / 5.3%;
[0079] M evaporation <15943.2-317.3*100% / 5.3% Kg / h;
[0080] That is, M evaporation <9956.4Kg / h;
[0081] Now, the sodium chloride component content is: M (NaCl) / (M total-M evaporation)*100%=2900 / (15943.2-9956.4)*100%=48.44%;
[0082] The solubility of sodium ch...
Embodiment 2
[0089] Example 2: Evaporation at 75°C
[0090] Solubility components of sodium chloride-sodium sulfate-water, as shown in Table 4:
[0091] Table 4 Solubility of sodium chloride-sodium sulfate-water at 75°C
[0092]
[0093] According to the mutual solubility relationship of sodium chloride-sodium sulfate at 75°C, the first-stage evaporation must control the sodium sulfate component to be less than 4.95%, while the sodium chloride component is greater than 25.25%, so that pure sodium chloride crystals can be precipitated. And sodium sulfate did not separate out. That is, the primary evaporation is:
[0094] M(Na 2 SO 4 ) / (M total-M evaporation)*100%<4.95%;
[0095] M evaporation 2 SO 4 )*100% / 4.95%;
[0096] M evaporation <15943.2-317.3*100% / 4.95% Kg / h;
[0097] That is, M evaporation <9533.1Kg / h;
[0098] Now, sodium chloride component content is:
[0099] M(NaCl) / (M total-M evaporation)*100%=2900 / (15943.2-9533.1)*100%=45.24%;
[0100] The solubility of sodium c...
Embodiment 3
[0107] Example 3: Evaporation at 100°C
[0108] Solubility components of sodium chloride-sodium sulfate-water, as shown in Table 5:
[0109] Table 5 Solubility of sodium chloride-sodium sulfate-water at 100°C
[0110]
[0111]
[0112] According to the mutual solubility relationship of sodium chloride-sodium sulfate at 100°C, the first-stage evaporation must control the sodium sulfate component to be less than 4.51%, and the sodium chloride component is greater than 25.9%, so that sodium chloride crystals can be precipitated, and Sodium sulfate did not precipitate out. That is, the primary evaporation is:
[0113] M(Na 2 SO 4 ) / (M total-M evaporation)*100%<4.51%;
[0114] M evaporation 2 SO 4 )*100% / 4.51%;
[0115] M evaporation <15943.2-317.3*100% / 4.51% Kg / h;
[0116] That is, M evaporation <8907.7Kg / h;
[0117] At this moment, the sodium chloride component content is:
[0118] M(NaCl) / (M total-M evaporation)*100%=2900 / (15943.2-8907.7)*100%=41.23%;
[0119] The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com