A phosphorylated peptide enrichment material and its preparation method and application
A phosphorylated peptide and enrichment technology, applied in the fields of material analysis chemistry and organic chemistry, can solve the problem that polyphosphorylated peptides are difficult to elute, non-specific adsorption of acidic peptide chains with basic residues phosphorylated peptides are difficult to retain, and cannot be achieved. Enrichment of phosphorylated peptides and other problems, to achieve the effect of easy reproducibility, improved detection limit and sensitivity, and selective enrichment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of phosphorylated peptide enrichment material
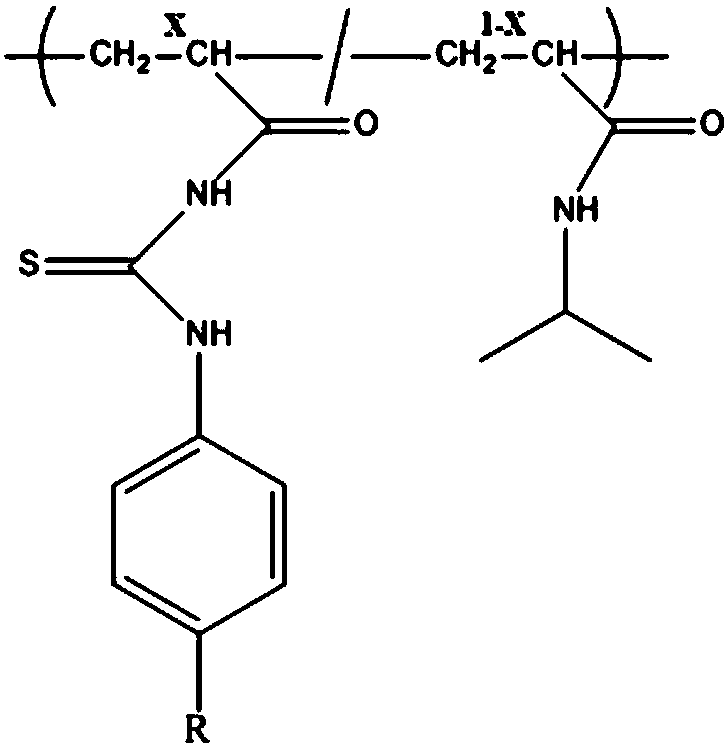
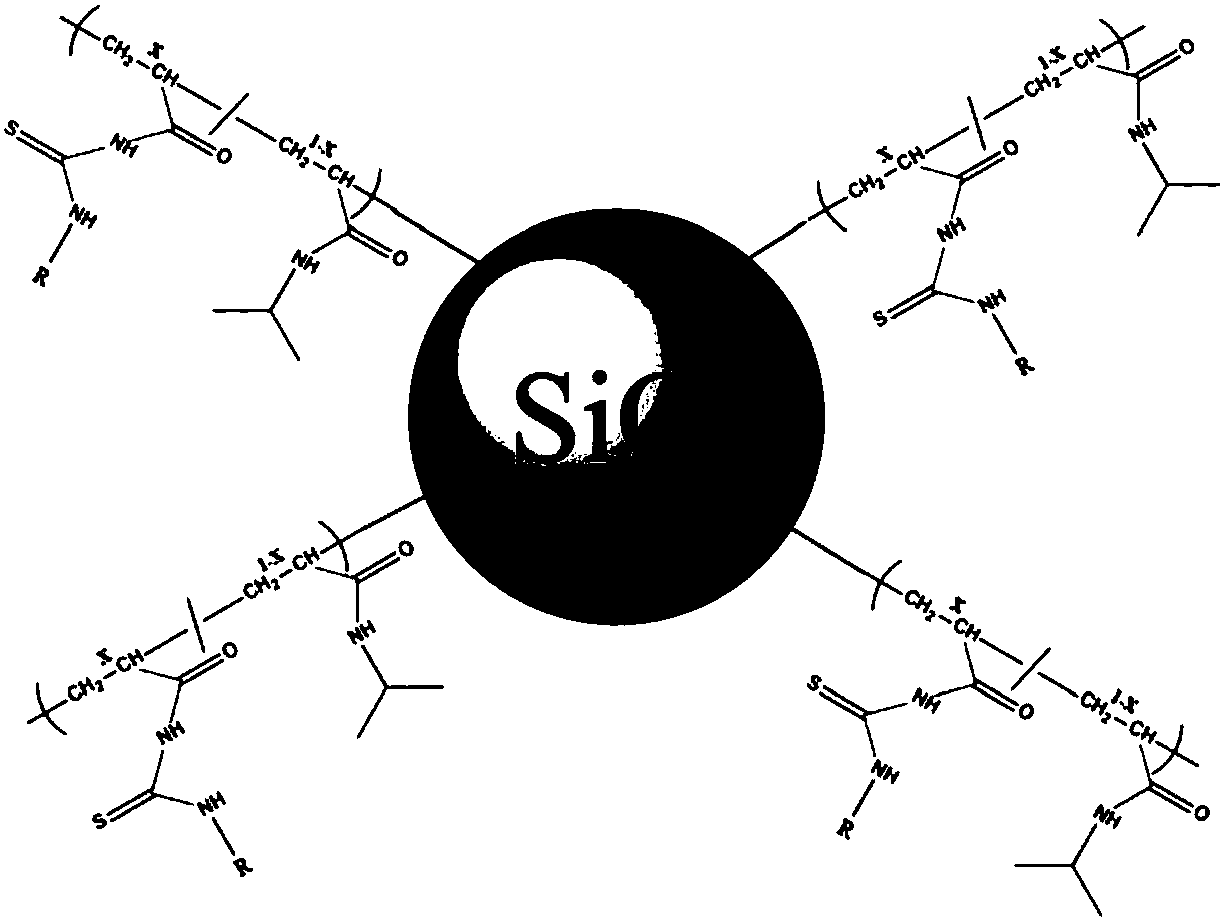
[0049] Two-component polymer structure such as figure 1 Shown, where X = 0.01-0.5. Taking X=0.2 as an example, add 0.4mmol of isopropylacrylamide and 0.1mmol of thiourea functional monomer into a 25ml three-necked flask in turn, the molar ratio of the substances is 4:1, and add 6mL of ultrapure water and 6mL of DMF as solvents at the same time ; Feed nitrogen under stirring, after the monomer is fully dissolved, add catalyst CuBr 0.032g and bipyridine ligand 0.16mL under nitrogen protection, then the reaction system is evacuated—nitrogen-filled to remove residual oxygen in the reaction system; Immerse the brominated substrate into the prepared reaction solution; control the temperature of the flask at 60-80°C and let it stand for 4-24 hours; after the reaction, use N,N'-dimethylformamide (DMF) and Deionized water (H 2 O) washing the surface of the polymer graft in sequence to obtain a phosphorylated peptide-...
Embodiment 2
[0052] Structure and Synthetic Method of Functional Monomer
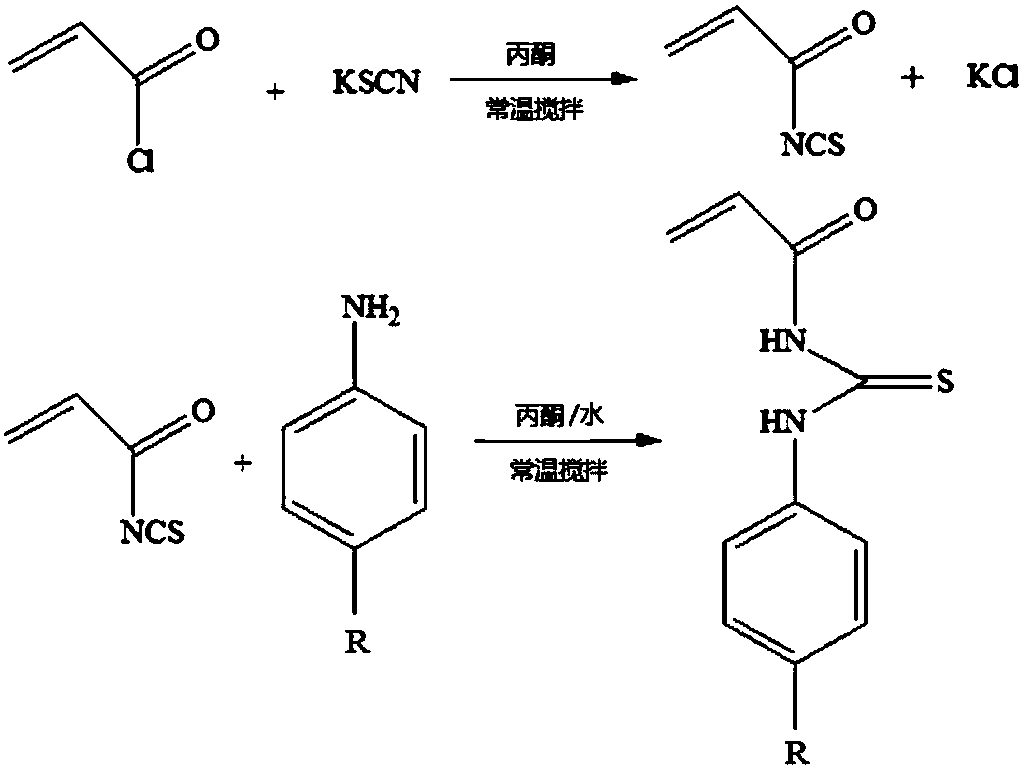
[0053] In order to prepare the above-mentioned two-component copolymer, a series of thiourea functional monomers need to be synthesized, and the synthetic method of their specific implementation is similar, and the synthetic steps are as follows: image 3 shown.
[0054] Taking the synthesis of thiourea monomer whose end group R is carboxyl as an example, dissolve 0.582g (6mmol) in 30-50mL of anhydrous acetone at room temperature, and add 0.453g (5mmol) of acryloyl chloride dropwise under stirring conditions. Add it dropwise to the above solution, and continue the reaction for 12 hours. Centrifuge (4500r / min, 5min) after the reaction, and take the supernatant for later use. At room temperature, dissolve 0.685g (6mmol) of p-aminobenzoic acid in 30mL of anhydrous acetone, add 2-4mL of ultrapure water, and add the spare clear solution dropwise to the above solution under stirring, and continue the reaction for 12 Ho...
Embodiment 3
[0061] The specific adsorption of phosphorylated peptides on the surface of two-component copolymers was evaluated by the method of QCM-D adsorption measurement, taking the thiourea monomer whose R terminal group is carboxyl group as an example. The two-component copolymer was grafted onto the surface of the QCM-D chip as described in Example 1, and the non-phosphorylated Peptides and mono-, di-, and tri-phosphorylated peptides were subjected to adsorption experiments, respectively. Figure 4 The adsorption curves of different peptide chains on the surface of the two-component copolymer are shown, which fully demonstrates the excellent specific adsorption of phosphorylated peptides of this type of polymer and the ability to effectively distinguish the number of phosphorylated sites. The field of enrichment and separation has very broad application prospects. 000000
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com