Rennin mutant with improved enzyme activity and thermal stability
A rennet and mutant technology, applied in the field of enzyme engineering, can solve the problems of decreased rennet activity, undiscovered research on bacterial-derived rennet site-directed mutation, etc., to reduce thermal stability, improve commercial value, and process Concise and clear effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Determine the site-directed mutation site of Bacillus amyloliquefaciens-derived chymosin gene
[0035] Simulate the tertiary structure of Mucor pumila chymosin gene by computer, and compare it with the tertiary structure and nucleic acid sequence of bovine chymosin, select three key sites for site-directed mutation, and select respectively those that may be related to hydrolysis or heat stability Sex-related loci 75, 81 and 222.
[0036] The 75th position was originally leucine, the 81st position was originally valine, and the 222nd position was originally methionine. The present invention pre-mutates the 75th position into glutamine, the 81st position into aspartic acid, and the 222nd position Point mutations to threonine were performed by a PCR-mediated one-step method.
[0037] Primers were designed for the selected mutation sites by DNAMAN, as shown in Table 1.
[0038] Table 1 Primer sequences used for site-directed mutagenesis
[0039]
[0040] (2) PCR-m...
Embodiment 2
[0049] (1) Expression of chymosin mutants in Pichia pastoris
[0050] For the expression steps of the chymosin mutant in Pichia pastoris, refer to the relevant operation steps in Example 1 of the patent application with application number 201611157248.4.
[0051] The recombinant vectors pPIC9K-cMCE-Leu75Gln, pPIC9K-cMCE-Val81Asp, pPIC9K-cMCE-Met222Thr, pPIC9K-cMCE-Leu75Gln-Val81Asp, pPIC9K-cMCE-Leu75Gln-Met222Thrp, and PIC9K-cMCEVal81Asphr were linearized with acIThrase , 2500V, 5ms electrotransformation of Pichia pastoris GS115 competent cells onto MM and MD plates successively, and His + and Mut + Type transformant P-cMCE.
[0052] Select transformants for colony PCR verification, perform agarose gel electrophoresis on the PCR products, select transformants in BMGY medium, and culture them at 30°C / 300rpm until OD600=2~6; collect the cells, resuspend the cells with BMMY, Make OD600=5.0 or so, add anhydrous methanol to the medium to a final concentration of 0.5% (v / v, 5mL m...
Embodiment 3
[0057] (1) Comparison of chymosin activity, protease activity and C / P value properties
[0058] The C / P value refers to the ratio of rennet activity to protease activity per unit mass of pure enzyme powder. It can effectively and accurately characterize the milk-clotting ability of rennet.
[0059] Mutant plasmids Leu75Gln, Val81Asp, Met222Thr, Leu75Gln-Val81Asp, Leu75Gln-Met222Thr, Val81Asp-Met222Thr were transformed into Pichia pastoris GS115, 30 transformants were randomly selected, and the inoculation amount was 0.5%. The starting strain P-cMCE without mutation was used as a control After BMGY culture and methanol induction, the pH is 6.5-6.7, the fermentation temperature is 30°C, and the fermentation is carried out in BMMY medium for 96 hours. The supernatant is collected by centrifugation, and the rennet activity and proteolytic activity of the crude enzyme liquid are measured, and the C / P ratio is calculated. The results are shown in Table 2.
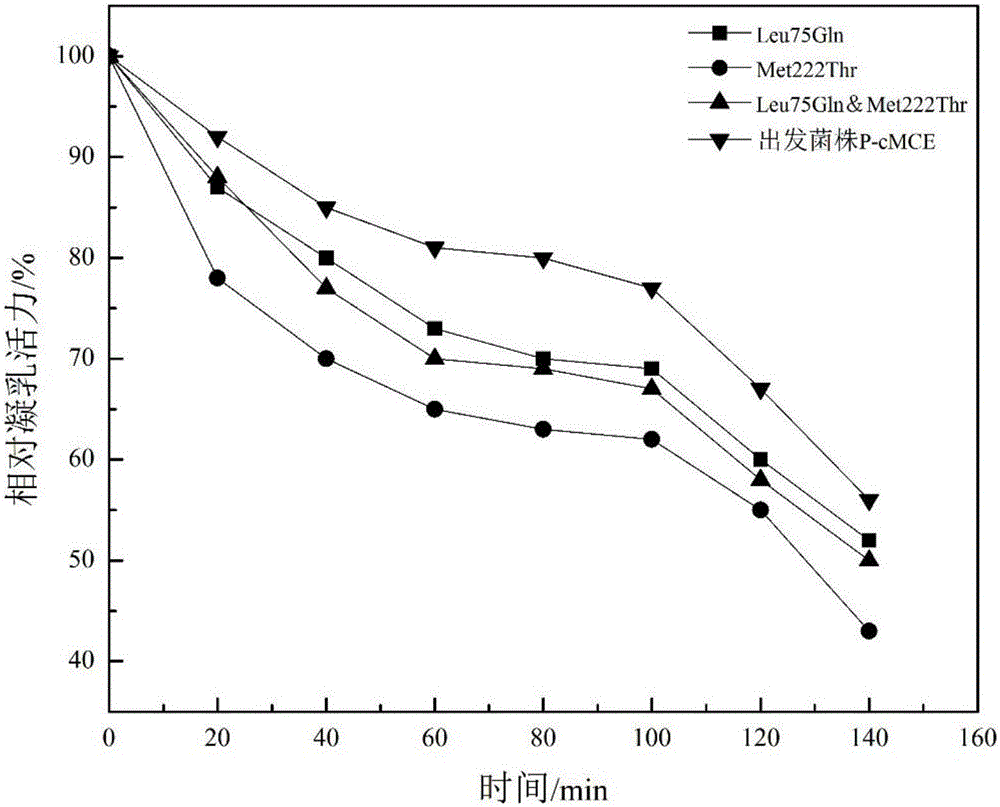
[0060] It can be seen f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com