Biological activity small peptide preparation method based on combined self-cleavage and protein scaffold
A technology of biological activity and protein scaffold, which is applied in the field of bioactive small peptide preparation based on combined self-shearing and protein scaffold, can solve the problem of damage to myofibrils, save time, simplify steps and costs, and the steps are simple and convenient Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
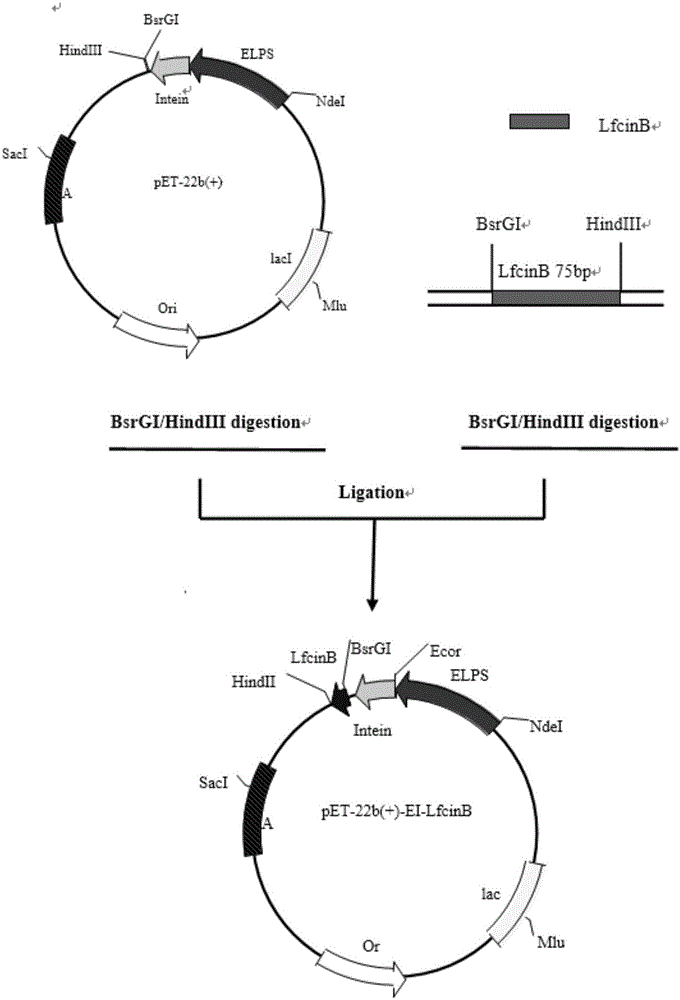
[0081] The construction of embodiment 1.ELPs-mediated C-intein fusion LfcinB recombinant plasmid
[0082] Using the existing pET-22b-EI-mCherry plasmid in the laboratory, the original mCherry gene was excised by restriction endonuclease, and replaced with the lfcinb, 2lfcinb and 4lfcinb genes amplified by PCR or whole gene synthesis to construct pET- 22b-EI-LfcinB, ET-22b-EI-2LfcinB, pET-22b-EI-4LfcinB three recombinant expression plasmids. Nucleic acid electrophoresis verification and sequencing results showed that the recombinant plasmid was successfully constructed.
[0083] 3 LfcinB gene sequence: Since the length of LfcinB is only 75bp, 25 amino acid residues, its amino acid sequence is FKCRRWQW RMKKLGAPSITCVRRAF (SEQ ID NO: 1), so two longer primers can be directly designed, and the target gene can be obtained by overlapping PCR. The primers were designed as follows:
[0084] LfcinB-F:
[0085] CAGCGT GTACA CAACATGTTCAAATGCCGTCGTTGGCAGTGGCG BSrGI (SEQ ID NO: 2...
Embodiment 2
[0102] Expression and purification of embodiment 2.EI-LfcinB
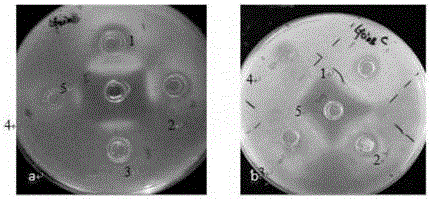
[0103] Expression of EI-LfcinB: After exploring the expression conditions of the target protein, it was found that among the three recombinant proteins EI-LfcinB, EI-2LfcinB, and EI-4LfcinB, only EI-LfcinB was effectively expressed, while EI-2LfcinB, EI -4LfcinB cannot be expressed in two different expression hosts. Therefore, only the expression conditions of EI-LfcinB were explored, and the LfcinB antimicrobial peptide was successfully purified. The expression and purification results of EI-LfcinB are as follows: Figure 6 shown.
[0104] Depend on Figure 6 It can be seen that EI-LfcinB is expressed in E.coli BL21(DE3), and the fusion protein EI-LfcinB can be obtained through two ITC cycles, with a size of about 67KD. However, this method also has some shortcomings. It can be seen from the figure that there is still a small amount of foreign protein in the fusion protein purified by ITC.
[0105]Purificatio...
Embodiment 4
[0107] Example 4. Optimization and purification of EI-LfcinB induced expression conditions
[0108] In order to increase the yield of EI-LfcinB, we explored and optimized the expression conditions of pET-22b-EI-LfcinB / E.coli BL21(DE3), and the results are shown below.
[0109] (1) EI-LfcinB adding IPTG concentration optimization
[0110] Five experimental groups were designed, cultivated in a constant temperature shaker at 37°C, 200rpm for 2-3h, and waited for OD 600 After reaching about 0.5, add IPTG with final concentrations of 0.2, 0.4, 0.6, 0.8, and 1.0 mM to each group of test tubes, and turn to 20°C to induce expression for 24 hours. The bacteria were directly collected for SDS-PAGE, and the results Figure 8 It shows that when the induction concentration of IPTG is 1.0mM, the expression of EI-LfcinB is the highest.
[0111] (2) EI-LfcinB adding IPTG to induce expression temperature optimization
[0112] Five experimental groups were designed, cultivated in a constan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com