Method for preparing high-multiplying-factor porous lithium manganate
A porous lithium manganate and high-rate technology, which is applied in the field of preparation of high-rate porous lithium manganate, can solve problems such as difficult large-scale production, cumbersome operation, and complicated preparation process, and achieve easy industrialization, simple equipment and process, and simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 16ml of DMF to a 100ml polytetrafluoroethylene stainless steel reaction kettle, weigh 4mmol of terephthalic acid into the reaction kettle, stir evenly, and then weigh 24mmol of MnCl 2 Add it into a reaction kettle, stir evenly, seal it, put it in an oven, heat at 150° C. for 48 hours, and filter and dry to obtain a metal-organic framework containing manganese. The manganese-containing metal-organic framework and lithium carbonate were uniformly mixed at a molar ratio of manganese and lithium elements of 2:1.05, and sintered at 650° C. for 10 hours to obtain porous lithium manganese oxide.
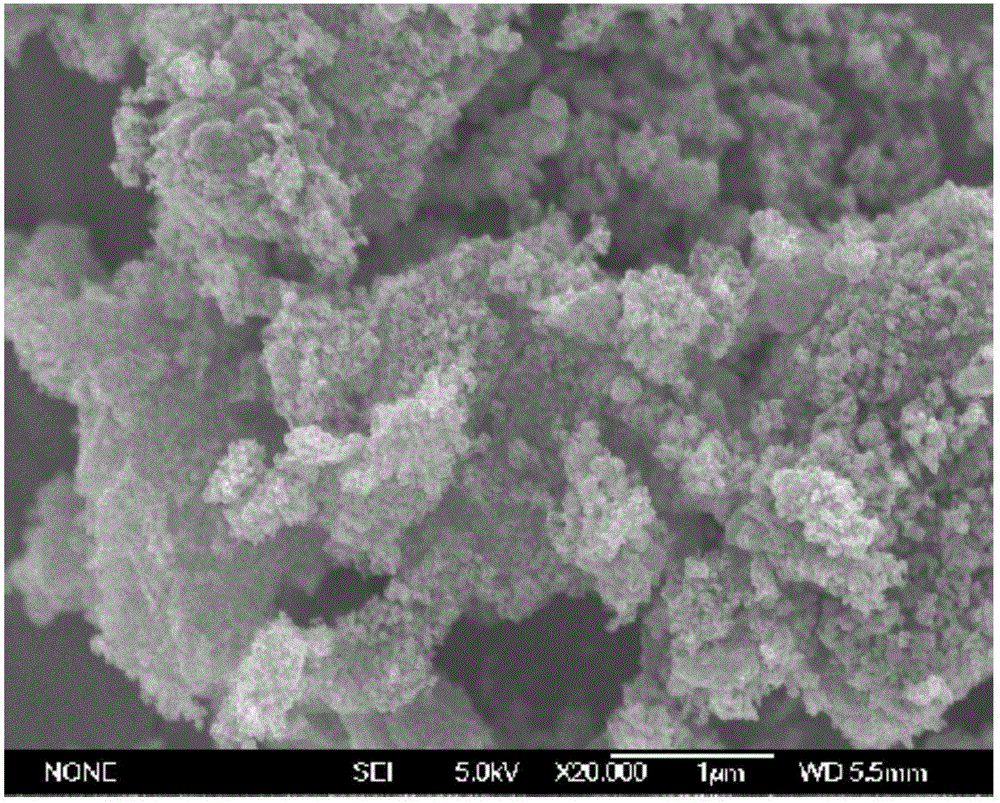
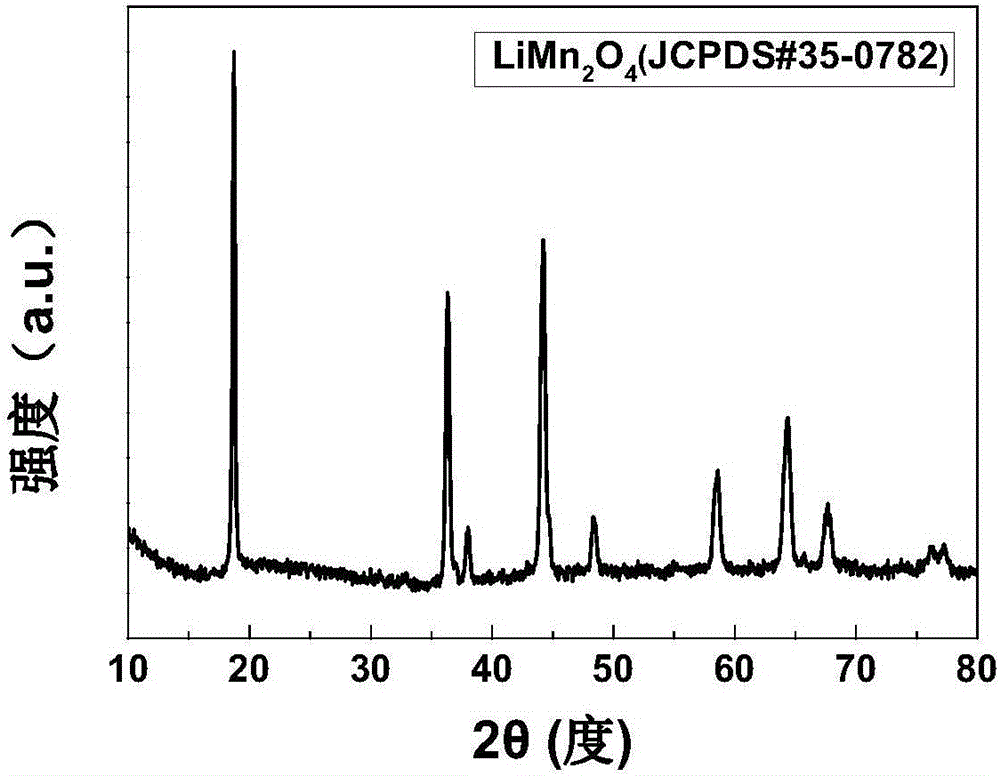
[0032] figure 1 SEM photo of porous lithium manganese oxide; figure 2 XRD picture of porous lithium manganese oxide.
[0033] From figure 1 It can be seen that the prepared lithium manganate is a porous structure, wherein the particle size of lithium manganate is 20-40 nanometers, from figure 2 It can be seen that its XRD is consistent with the standard card.
Embodiment 2
[0035] Add 8ml of DMF to a 50ml polytetrafluoroethylene stainless steel reaction kettle, weigh 2mmol of terephthalic acid and add it to the reaction kettle, stir evenly, and then weigh 24mmol of MnCl 2 Add it into a reaction kettle, stir evenly, seal it, put it in an oven, heat it at 120° C. for 72 hours, filter and dry it, and obtain a metal-organic framework containing manganese. The manganese-containing metal-organic framework and lithium hydroxide were uniformly mixed at a molar ratio of manganese and lithium elements of 2:1, and sintered at 850° C. for 10 hours to obtain porous lithium manganate.
Embodiment 3
[0037] Add 8ml of DMF to a 50ml polytetrafluoroethylene stainless steel reaction kettle, weigh 2mmol of terephthalic acid and add it to the reaction kettle, stir evenly, and then weigh 24mmol of MnCl 2 Add it into a reaction kettle, stir evenly, seal it, put it in an oven, heat it at 180° C. for 24 hours, filter and dry it, and obtain a metal-organic framework containing manganese. The manganese-containing metal-organic framework and lithium hydroxide were uniformly mixed at a molar ratio of manganese to lithium of 2:1.1, and sintered at 600°C for 5 hours to obtain porous lithium manganese oxide.
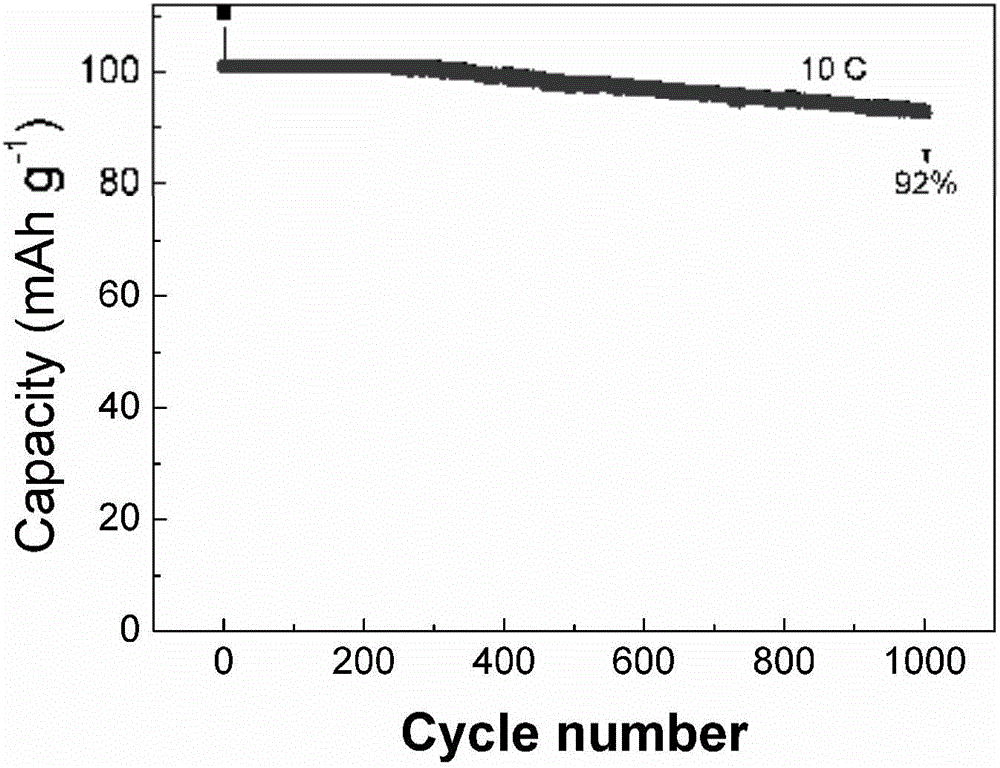
[0038] Weigh 0.2 g of the final product, mix it with carbon black and PVDF at a mass ratio of 8:1:1, and stir it thoroughly with NMP as a solvent. After mixing evenly, apply it to aluminum foil and dry it in vacuum at 100°C for 12 hours. After cutting, pack it into a battery in a glove box, and do a high-rate performance test on a charge-discharge instrument. image 3 It is the hig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com