Preparation method for L-oxiracetam oral preparation
Another kind, the technology of oral film, is applied in the field of preparation of oral preparations of levoxiracetam, which can solve the problems of needing further research, unfavorable taking, unsuitable for large-scale production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
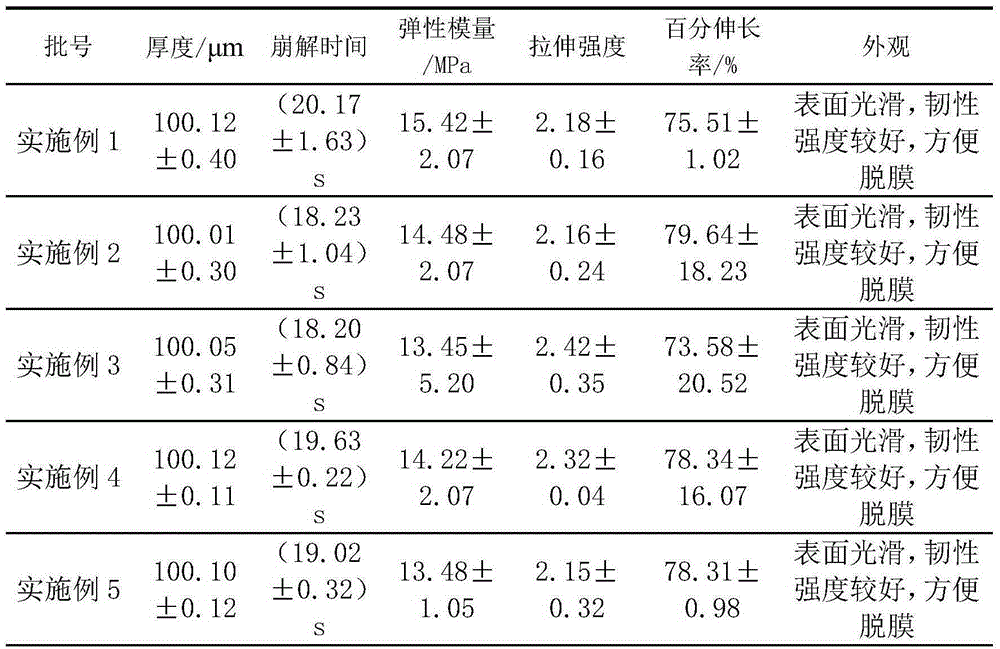
Embodiment 1
[0082] The preparation of L-Oxiracetam film is as follows:
[0083] 1) Dissolve 60g of film-forming material (composed of acrylate and hydroxypropylmethylcellulose, of which 30g of hydroxypropylmethylcellulose) is dissolved in 80mL of absolute ethanol, and remove bubbles to obtain a uniform viscous liquid;
[0084] 2) Disperse 18 g of dibutyl phthalate and 15 g of microcrystalline cellulose with 50 mL of absolute ethanol to form a dispersion;
[0085] 3) Add the dispersion of step 2) to the viscous liquid of step 1), and add 7g of levoxiracetam to disperse evenly, and then let it stand to remove air bubbles;
[0086] 4) Coat the viscous liquid after the bubbles have been removed with a medical film coating film dryer at a coating speed of 50cm / min, then dry at 65-68°C, peel off and cut it.
Embodiment 2
[0088] The preparation of L-Oxiracetam film is as follows:
[0089] 1) Dissolve 50g of the film-forming material (composed of acrylate and hydroxypropylmethylcellulose, of which the amount of hydroxypropylmethylcellulose is 10g) with 50mL of absolute ethanol, and remove bubbles to obtain a uniform viscous liquid;
[0090] 2) Disperse 12 g of triacetin and 20 g of low-substituted hydroxypropyl cellulose with 60 mL of absolute ethanol to form a dispersion;
[0091] 3) Add the dispersion of step 2) to the viscous liquid of step 1), and add 18 g of levoxiracetam to disperse evenly, and then let it stand to remove air bubbles;
[0092] 4) The viscous liquid after removing the bubbles is coated with a film coating dryer at a coating speed of 80cm / min, then dried at 70-72°C, peeled and cut.
Embodiment 3
[0094] The preparation of L-Oxiracetam film is as follows:
[0095] 1) Dissolve 55 g of film-forming material (composed of acrylate and hydroxypropyl methyl cellulose, of which 20 g of hydroxypropyl methyl cellulose) is dissolved in 50 mL of absolute ethanol, and remove bubbles to obtain a uniform viscous liquid;
[0096] 2) Disperse 15g of trimethyl citrate and 15g of microcrystalline cellulose with 40mL of absolute ethanol to form a dispersion;
[0097] 3) Add the dispersion liquid of step 2) to the viscous liquid of step 1), and add 15 g of levoxiracetam to disperse evenly, and then stand to remove air bubbles;
[0098] 4) Coat the viscous liquid after the bubbles have been removed with a medical film coating film dryer at a coating speed of 70cm / min, then dry it at 80-85°C, peel it off, and cut it.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com