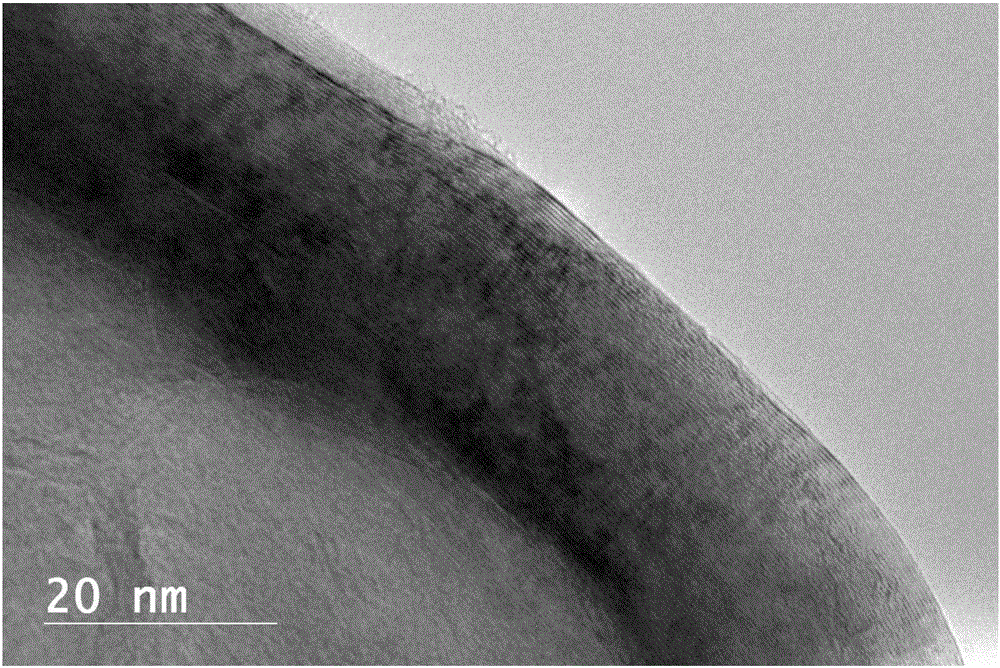
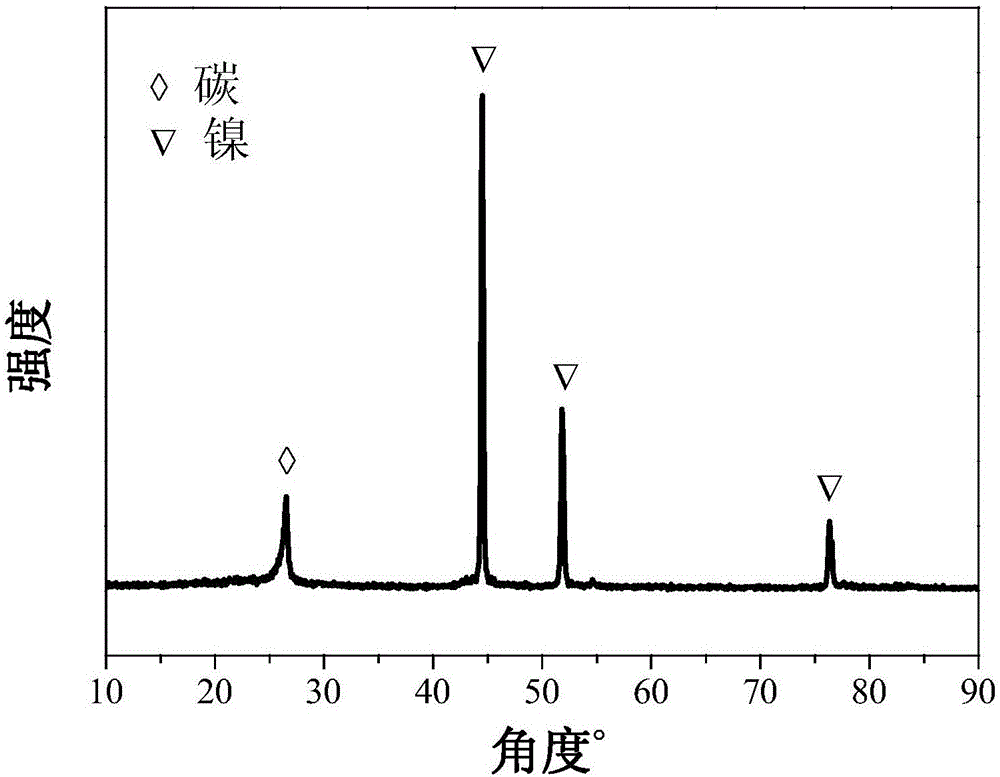
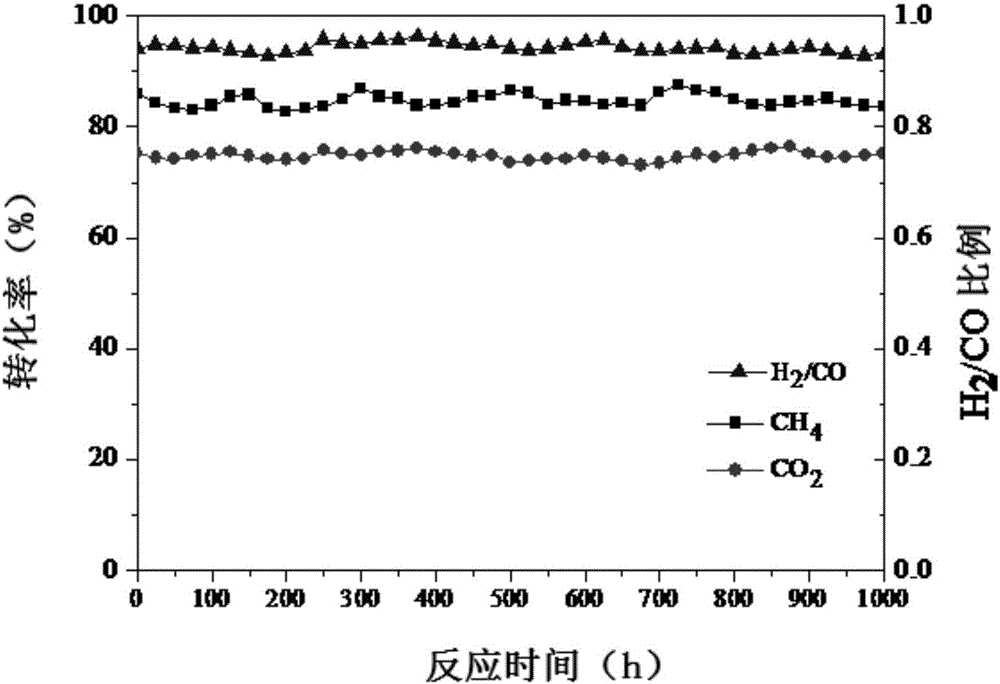
Carbon loaded nickel metal catalyst and preparation method thereof
A metal catalyst and nickel-supported technology, which is applied in the direction of metal/metal oxide/metal hydroxide catalyst, carbon preparation/purification, chemical instruments and methods, etc., can solve the problems of inability to recycle, waste of resources, and catalyst deactivation and other issues, to achieve the effect of saving resources, good stability and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] A method for preparing a carbon-supported nickel metal catalyst, comprising the steps of:
[0041] Step 1. Pretreatment of carbon source: mix carbon source with 0.1-1mol / L nitric acid, sulfuric acid or phosphoric acid solution at a ratio of 200-350g:400-1000mL, and reflux at a temperature of 70-85°C for 6-12 hours Filtrate, wash the filter residue with deionized water until pH=7; then dry at 100-110°C for 8-12 hours to obtain solid A, keep solid A at 150-180°C for 12-24 hours, wash, After drying at ℃ for 8-12 hours, solid B was obtained;
[0042] Step 2. Preparation of nickel-based catalyst precursor: dissolve the nickel-containing compound in deionized water, stir until completely dissolved to obtain solution C, and then add solid B or carbon source to solution C equal in volume or amount to B or carbon source After standing at room temperature for 12-24 hours, dry at 100-110°C for 8-12 hours to obtain solid D;
[0043]Step 3. Preparation of carbon-supported nickel m...
Embodiment 1
[0050] Catalyst preparation:
[0051] (1) Take 250g of starch and put it into a round bottom flask containing 1000mL of 0.1mol / L nitric acid solution, reflux for 10h at a temperature of 70°C and a rotation speed of 900RPM, then wash with deionized water until the supernatant pH=7, then Dry at a temperature of 100° C. for 12 h to obtain solid A. A was subjected to hydrothermal treatment at a temperature of 180° C. for 24 hours, washed, and dried at 100° C. for 12 hours to obtain solid B.
[0052] (2) Dissolve 36 g of nickel nitrate hexahydrate in 200 mL of deionized water and stir until completely dissolved to obtain solution C. Then 20 g of solid B obtained in step (1) was added to solution C, and after standing at room temperature for 24 hours, it was dried at 100° C. for 12 hours to obtain solid D.
[0053] (3) Take 300 mg of solid D obtained in step (2) and send it into a quartz reaction tube with an outer diameter of 1 inch, and use a program temperature control heating ...
Embodiment 2
[0057] Catalyst preparation:
[0058] (1) Take 250g of starch and put it into a round bottom flask filled with 800mL 0.5mol / L nitric acid solution, reflux for 8 hours at a temperature of 75°C and a rotation speed of 800RPM, then wash with deionized water until the supernatant pH=7, then Dry at a temperature of 100° C. for 12 h to obtain solid A. A was subjected to hydrothermal treatment at a temperature of 150°C for 24 hours, washed, and dried at 100°C for 12 hours to obtain solid B.
[0059] (2) Dissolve 42 g of nickel nitrate hexahydrate in 200 mL of deionized water, and stir until completely dissolved to obtain solution C. Then 20 g of solid B obtained in step (1) was added to solution C, and after standing at room temperature for 24 hours, it was dried at 100° C. for 12 hours to obtain solid D.
[0060] (3) Get 300 mg of the solid D obtained in step (2) and send it into a quartz reaction tube with an outer diameter of 1 inch, and use a program temperature control to heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com