Analysis method of disaccharides containing uronic acid based on reduction amine derivatization and preparation method thereof
A technology of uronic acid disaccharide and analysis method, which is applied in the analysis method and preparation field of uronic acid disaccharide-containing uronic acid disaccharide based on derivatization of reductive amine, can solve the problems of subtle difference in molecular structure, large polarity of oligosaccharide, etc. Removal, simple operation, chemically stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
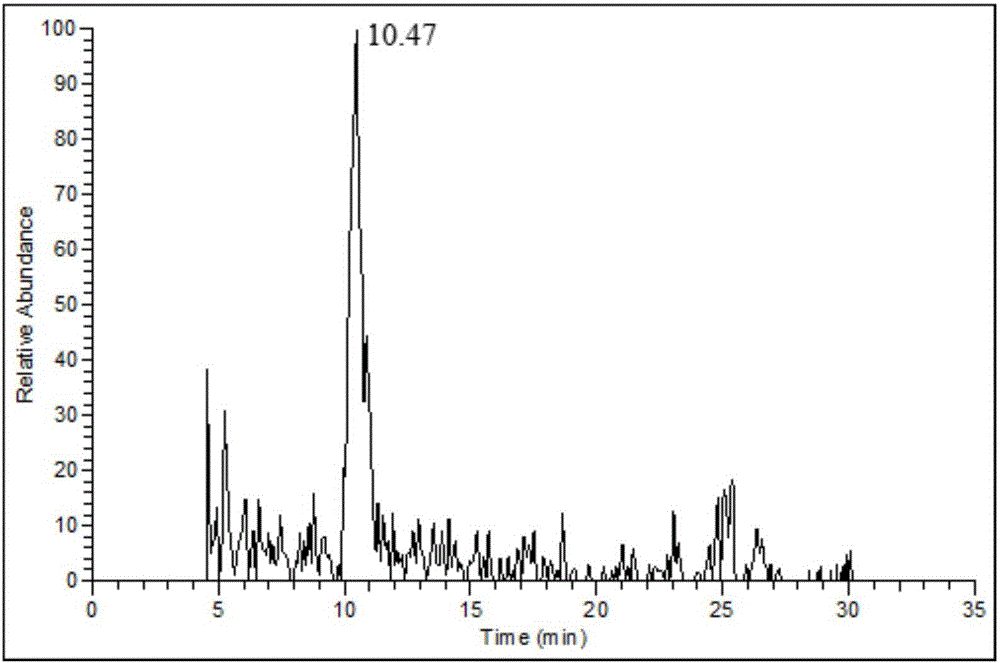
[0054] The invention provides an analysis method and preparation method based on derivatization of uronic acid-containing disaccharides based on reducing amines. The specific steps are as follows:
[0055] An analytical method based on derivatization of uronic acid disaccharides based on reducing amines:
[0056] S1. The polysaccharide in the sample is extracted by alcohol precipitation after enzymatic hydrolysis by double enzyme method;
[0057] S2. Dissolve the polysaccharide sample in water, treat with anion exchange resin, elute with 1.3M and 3M NaCl solution respectively, and then ultrafilter the collected eluate with ultrafiltration membrane (molecular weight cut-off: 3000-10000Da) Desalting, the intercepted part is acidic polysaccharide, and finally freeze-dried to obtain uronic acid-containing polysaccharide.
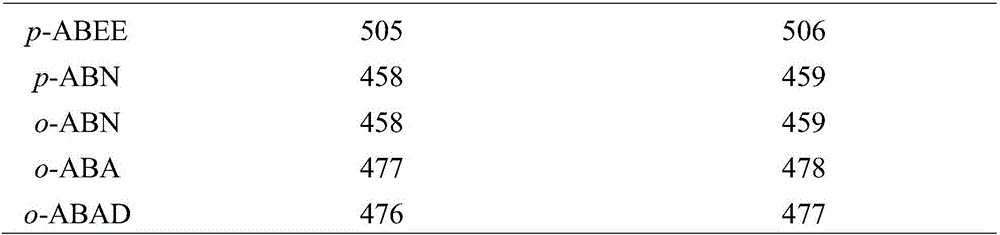
[0058] S3, dissolving the uronic acid-containing polysaccharide obtained in S2 in 1.3M trifluoroacetic acid solution, hydrolyzing at 105°C for 3 hours; adding ...
Embodiment 1
[0071] (1) Wash, shell and crush fresh green clams (Cyclina sinensis), take 10 g and place them in 100 mL of 0.05 mol / L phosphate buffer solution with pH=8, add 25 mL of 0.05 mol / L cysteine- EDTA-2Na solution, then add 0.5% (m / m) trypsin, 37 ° C water bath shaking enzymolysis for 4 hours, then add 0.5% (m / m) papain 65 ° C water bath shaking enzymolysis 3 h. Then inactivate the enzyme at 100°C for 5 min. Centrifuge at 12300×g for 20min. Take the supernatant, add 1.5 times of absolute ethanol to it, ethanol precipitate overnight, centrifuge at 7800×g for 15min, and take the precipitate. Ethanol was evaporated to dryness, and deionized water was added to redissolve, and then freeze-dried at low temperature to obtain crude polysaccharide.
[0072] (2) Accurately weigh 2.0g of crude polysaccharide dry powder and dissolve in 50mL water, handle with strong anion exchange resin, elute with the NaCl solution of 1.3M and 3M respectively, then use ultrafiltration membrane (molecular we...
Embodiment 2
[0082] (1) Wash, shell and crush fresh green clams (Cyclina sinensis), take 10 g and place them in 100 mL of 0.05 mol / L phosphate buffer solution with pH=8, add 25 mL of 0.05 mol / L cysteine- EDTA-2Na solution, then add 0.5% (m / m) trypsin, 37 ° C water bath shaking enzymolysis for 4 hours, then add 0.5% (m / m) papain 65 ° C water bath shaking enzymolysis 3 h. Then inactivate the enzyme at 100°C for 5 min. Centrifuge at 12300×g for 20min. Take the supernatant, add 1.5 times of absolute ethanol to it, ethanol precipitate overnight, centrifuge at 7800×g for 15min, and take the precipitate. Ethanol was evaporated to dryness, and deionized water was added to redissolve, and then freeze-dried at low temperature to obtain crude polysaccharide.
[0083] (2) Accurately weigh 2.0g of crude polysaccharide dry powder and dissolve in 50mL water, handle with strong anion exchange resin, elute with the NaCl solution of 1.3M and 3M respectively, then use ultrafiltration membrane (molecular we...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com