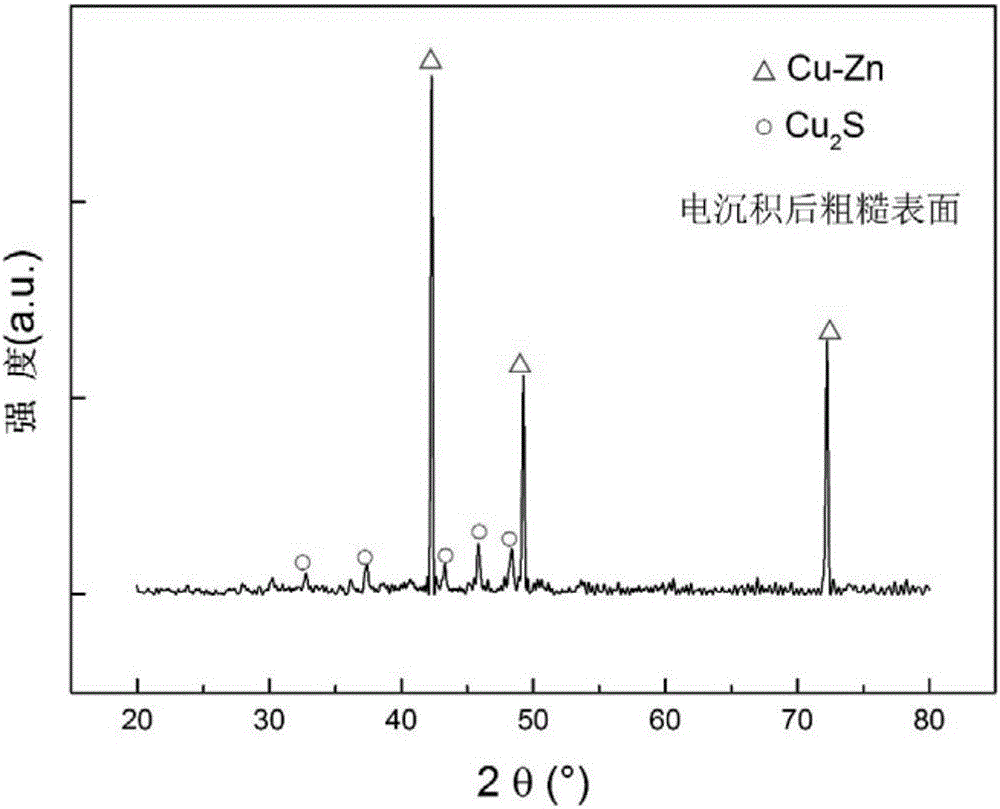
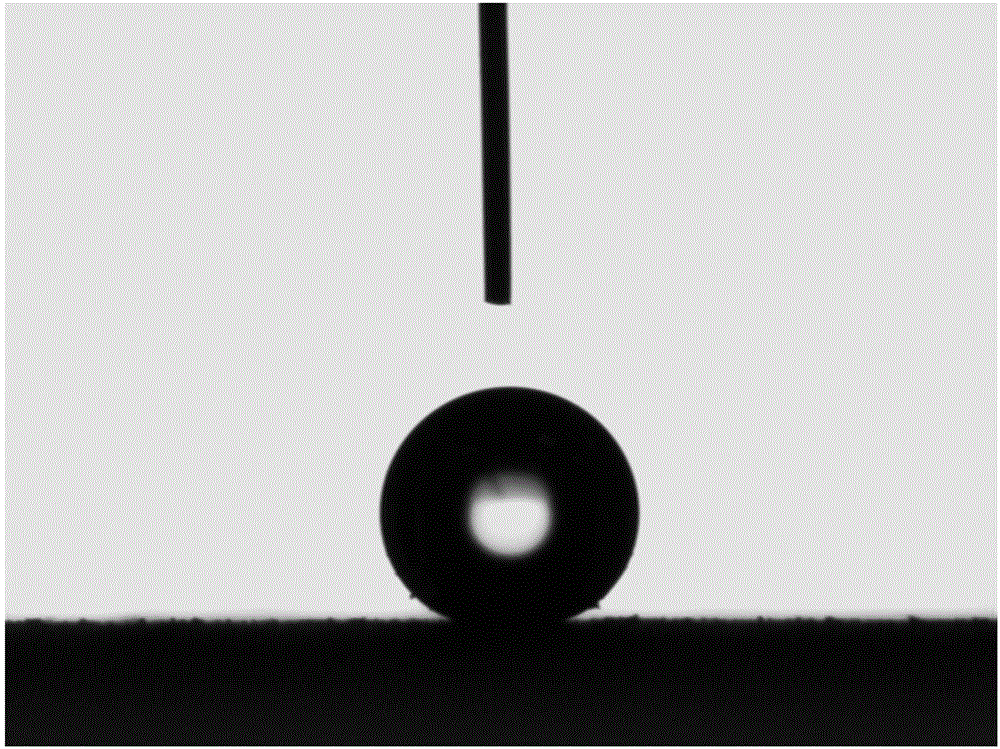
Preparation method for surface-wettability-controllable super-hydrophobic copper and alloy thereof
A technology of surface infiltration and copper alloy, which is applied to the surface coating liquid device, special surface, surface reaction electrolytic coating, etc., can solve the problems of potential safety hazards, high cost, and limited promotion, etc., to shorten the preparation cycle, The effect of controllable wettability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A red copper sheet with a size of 50×30 is electro-deposited after surface pretreatment and self-assembled with myristic acid to obtain a super-hydrophobic surface with outstanding structural features, specifically including the following steps and process conditions:
[0033] (1) Copper sheet pretreatment: ultrasonically clean the copper sheet with acetone, alcohol, and deionized water for 5 minutes at room temperature, and blow dry with a hair dryer to remove oil and impurities on the surface;
[0034] (2) Plating solution configuration: dissolve thiourea and disodium edetate in deionized water, wherein the concentration of thiourea is 0.05mol / L, and the concentration of disodium edetate is 0.01mol / L;
[0035] (3) Electrodeposition: take two pretreated copper sheets as two poles of alternating current respectively, take 100ml of the electroplating solution prepared in step (2) and conduct electrodeposition for 5min under the condition of alternating current voltage 5V,...
Embodiment 2
[0040] A brass sheet with a size of 50×30 is electro-deposited after surface pretreatment and self-assembled with myristic acid to obtain a super-hydrophobic surface with outstanding structural features, specifically including the following steps and process conditions:
[0041] (1) Copper sheet pretreatment: ultrasonically clean the brass sheet with acetone, alcohol, and deionized water for 10 minutes at room temperature, and blow dry with a hair dryer to remove oil and impurities on the surface;
[0042] (2) Plating solution configuration: dissolve thiourea and disodium edetate in deionized water, wherein the concentration of thiourea is 0.3mol / L, and the concentration of disodium edetate is 0.2mol / L;
[0043] (3) Electrodeposition: take two pretreated copper sheets as two poles of alternating current respectively, take 100ml of the electroplating solution prepared in step (2) and conduct electrodeposition for 10min under the condition of alternating current voltage 20V, and ...
Embodiment 3
[0048]Phosphor copper sheets with a size of 50×30 are electro-deposited after surface pretreatment and self-assembled with myristic acid to obtain a super-hydrophobic surface with outstanding structural features, specifically including the following steps and process conditions:
[0049] (1) Copper sheet pretreatment: ultrasonically clean the phosphor copper sheet with acetone, alcohol, and deionized water for 2 minutes at room temperature, and blow dry with a hair dryer to remove oil and impurities on the surface;
[0050] (2) Plating solution configuration: dissolve thiourea and disodium edetate in deionized water, wherein the concentration of thiourea is 0.2mol / L, and the concentration of disodium edetate is 0.5mol / L;
[0051] (3) Electrodeposition: take two pretreated copper sheets as the poles of alternating current, take 100ml of the electroplating solution prepared in step (2) and conduct electrodeposition for 2min under the condition of AC voltage 40V, and the temperatu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com