Oxygen evolution reaction electrocatalyst and preparation method thereof
A technology of electrocatalyst and oxygen evolution reaction, which is applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. High potential and other problems, to achieve the effect of good dispersion, simple equipment, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
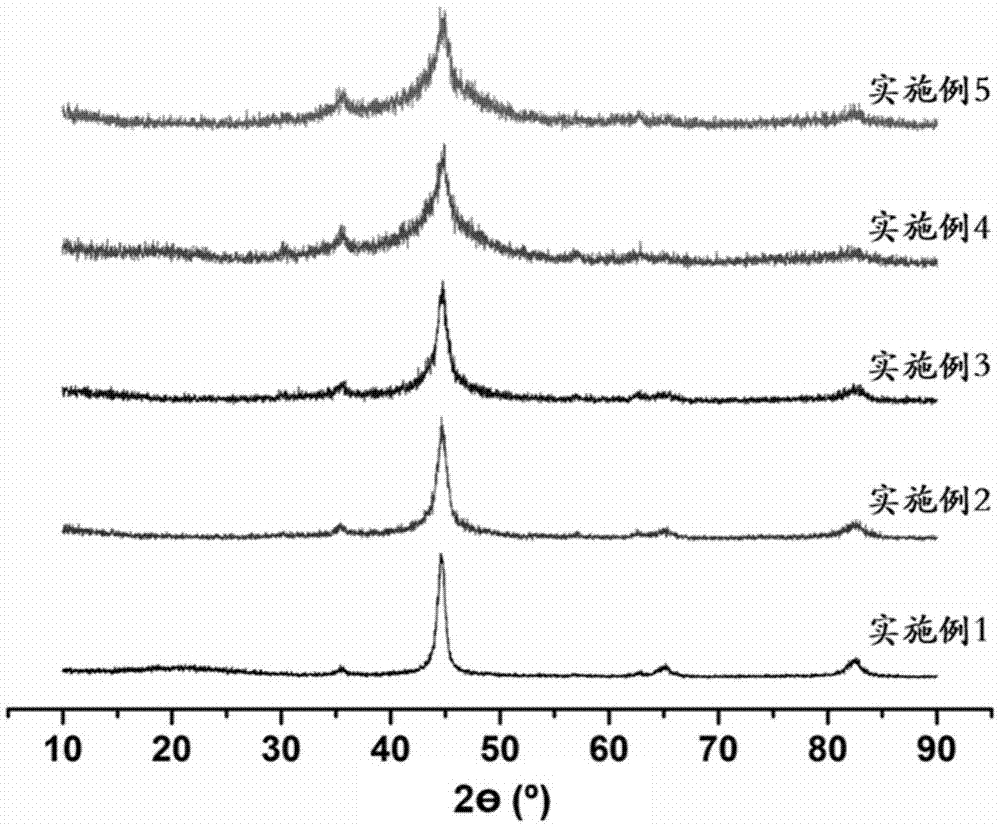
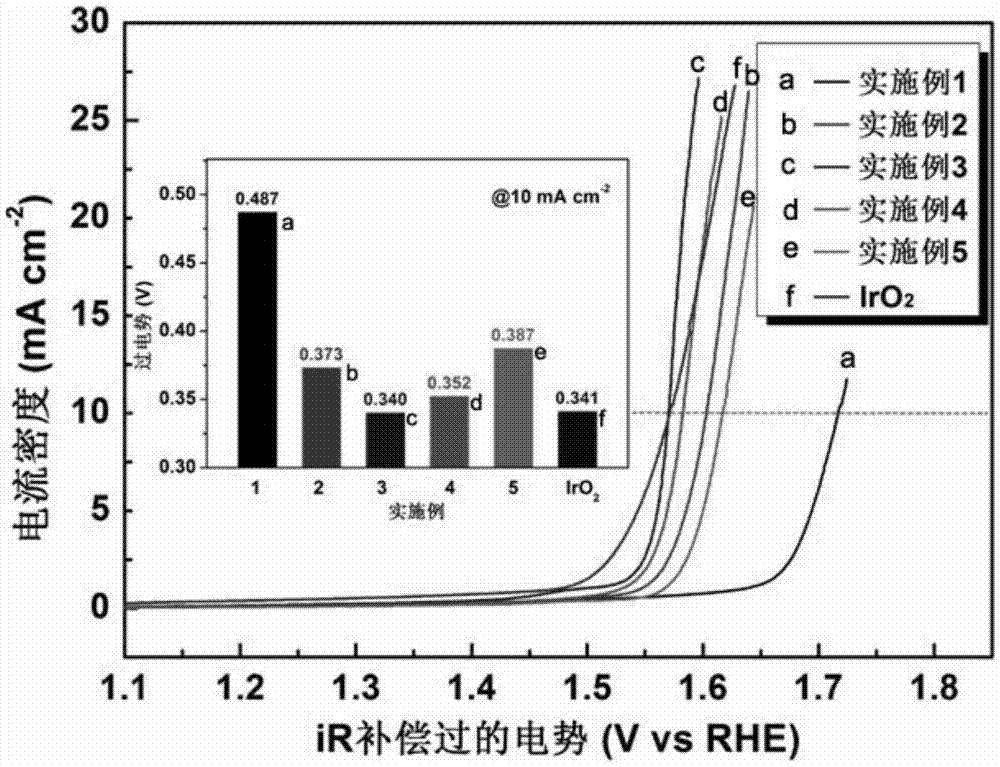
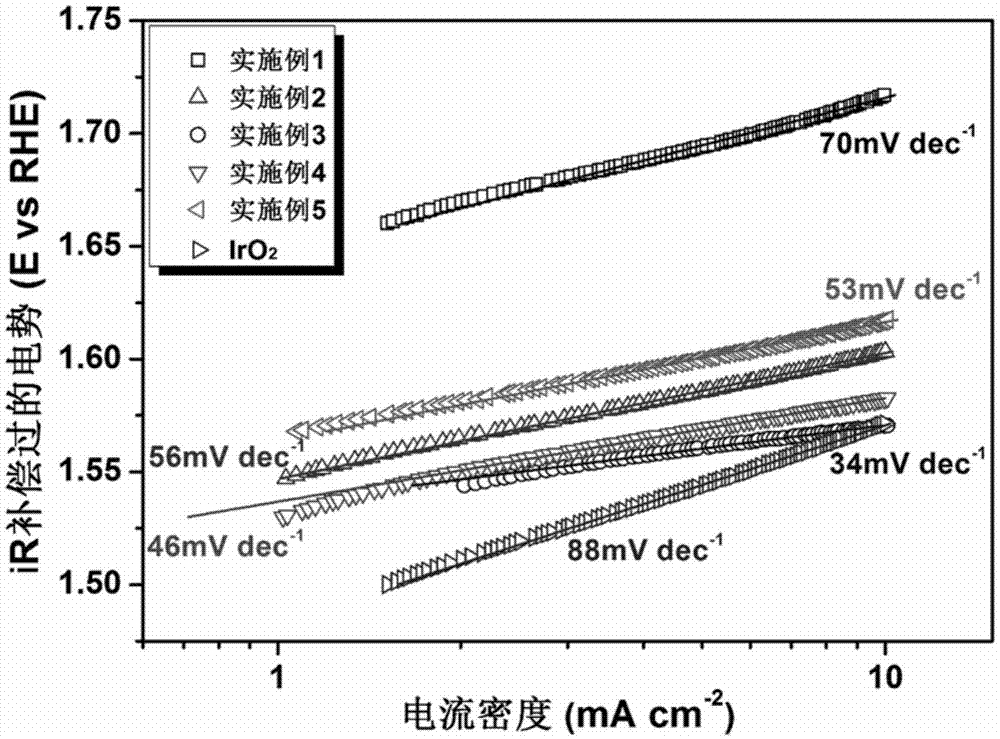
Image
Examples
preparation example Construction
[0014] The preparation method of the electrocatalyst for the oxygen evolution reaction provided by the present invention is that without the protection of an inert gas, under the protection condition of an ice-water bath, sodium borohydride (NaBH 4 ) solution is quickly added to the solution containing ferrous ions or mixed metal ions in the solution I reaction technical scheme. The technical scheme adopts the liquid phase reduction method to prepare nanometer zero-valent nickel-iron particles. Under the protection of no inert gas, the ferrous salt or the mixed metal salt of ferrous salt and nickel salt is dissolved in distilled water, stirred and mixed evenly, and placed in an ice water bath. Under the protection, the corresponding amount of NaBH 4 The solution is quickly poured into the salt solution, the color of the solution turns black immediately, and the liquid phase reduction reaction is carried out under stirring conditions for about 20-35 minutes, and the ferrous ion...
Embodiment approach
[0022] (1) ice-water bath protection, nickel chloride and ferrous sulfate heptahydrate are dissolved in water to prepare solution I;
[0023] In solution I, the total concentration of nickel ions and ferrous ions is 0.14mol / L to 0.16mol / L, and the molar ratio of nickel ions to ferrous ions is 0 to 0.4; preferably 0 to 0.3; more preferably 0.05 to 0.2; most preferably 0.1.
[0024] (2) Pour the sodium borohydride aqueous solution whose concentration is 0.7mol / L~1.1mol / L into the above-mentioned solution I rapidly, make the amount of the substance of sodium borohydride be 5~7 times of the metal ion total amount in the solution I; Continue to stir and react for 20-35 minutes; the obtained product is first washed with deionized water to pH = 7, then washed with absolute ethanol, dispersed in acetonitrile after centrifugal separation, and dried in a low-temperature freezer.
[0025] An oxygen evolution reaction electrocatalyst further provided by the present invention is prepared ...
Embodiment 1
[0039] Weigh 0.83 g of ferrous sulfate heptahydrate, dissolve it in 20 ml of water, and protect it in an ice-water bath. The concentration of ferrous sulfate is 0.15 moles per liter. Quickly pour 20 milliliters of 0.9 mol per liter sodium borohydride solution into the ferrous sulfate solution, and continue to stir and react for 30 minutes. Wash with water until pH = 7, then wash once with absolute ethanol and centrifuge. Disperse the product in acetonitrile, and dry it in vacuum at low temperature in a freeze dryer. Metallic Fe particles with a size of 10-100 nm were obtained. The obtained black powder and 5 mg of XC-72 were weighed and dispersed in 2 mL of isopropanol, then 50 μL of Nafion (5%) solution was added, and ultrasonically oscillated for 30 min to mix uniformly to obtain a catalyst slurry. Then pipette 20 μL of the catalyst slurry onto a rotating disk electrode with a diameter of 5 mm, and let it dry naturally in the air. The obtained electrode was evaluated for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com