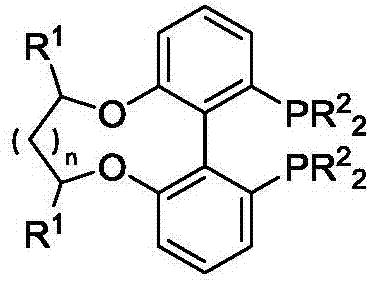
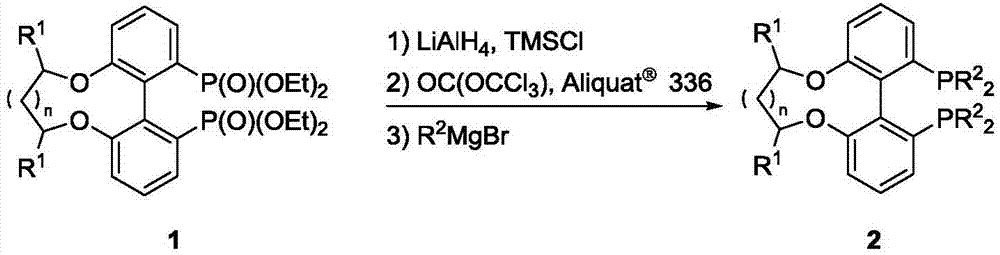
Center chirality-axial chirality electron deficiency diphosphine ligand as well as synthesis and application thereof
A bisphosphine ligand and electron-deficient technology is applied in the synthesis field of central chirality-axial chirality electron-deficient bisphosphine ligands, and achieves the effects of fewer reaction steps, simple operation, and fewer separation and purification steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0032] Example: Synthesis of bis(3,4,5-trifluorophenyl)phosphine ligand (2a)
[0033]
[0034] Under the protection of nitrogen, the LiAlH 4 (455mg, 12mmol) and THF (10mL) were added to a 100mL Schlenk flask, the temperature was cooled to -40℃, TMSCl (1.1mL, 12mmol) was added dropwise, after the addition, the mixture was stirred at low temperature for 0.5 hours, then 20mL of 1a THF solution was added dropwise, and the temperature was slowly increased. To room temperature, react for 3 hours, slowly add 7 mL of degassed methanol, and add 6 mL of 30% NaOH degassed aqueous solution. The reaction solution is filtered, separated, washed with degassed water under the protection of nitrogen, and then separated again with anhydrous sodium sulfate. Dry, extract with degassed ether, vacuum cold hydrazine to drain the solvent, and obtain a brownish yellow viscous as phosphine hydrogen compound.
[0035] Under the protection of nitrogen, add triphosgene (1.187g, 4mmol) into the pressure tube, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com