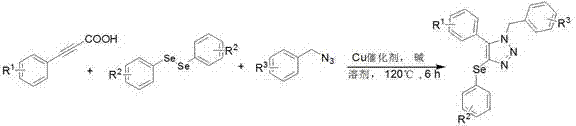
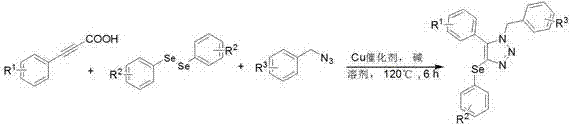
Method for synthesizing selenium-containing triazole compound by using diselenide, acetylenic acid and nitrine
A synthesis method and technology of diselenide, applied in the field of synthesizing selenium-containing triazole compounds, can solve the problems of limited organic selenium sources, low reaction yield, complex extraction process, etc., achieve good application prospects, simple operation, The effect of easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1-Benzyl-5-phenyl-4-(phenylselenyl)-1 H Synthesis of -1,2,3-triazole:
[0018] Add 0.5 mmol of phenylpropylic acid, 0.5 mmol of diphenyldiselenide, 0.5 mmol of benzyl azide, 10 mol% anhydrous copper sulfate, 0.6 mmol of potassium carbonate and 3 mL of toluene to the sealed tube, at 120 o The reaction was carried out at C for 6 h, and the reaction was monitored by TLC; after the reaction was complete, it was cooled to room temperature, the solvent was removed under reduced pressure, and purified by flash silica gel column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain the yellow solid product 1a, the product The rate is 80%, and its structural formula is, ;
[0019] Product characterization: 1 H NMR (400 MHz, CDCl 3 ) δ 8.06 – 8.04 (m, 2H), 7.43 – 7.33 (m,3H), 7.23 – 7.21 (m, 5H), 7.18 – 7.08 (m, 3H), 7.01 – 6.94 (m, 2H), 5.66 (s ,2H). 13 C NMR (100 MHz, CDCl 3 ) δ 151.5, 134.8, 130.5, 129.6, 129.5, 129.2, 128.6, 128.5, 128.4, 128.1, 127.9, 127...
Embodiment 2
[0021] 1-Benzyl-5-(4-methylphenyl)-4-(phenylselenyl)-1 H Synthesis of -1,2,3-triazole:
[0022] Add 0.5 mmol p-tolylpropiolic acid, 0.5 mmol diphenyldiselenide, 0.5 mmol benzyl azide, 10 mol% anhydrous copper acetate, 0.6 mmol potassium hydroxide and 3 mL xylene at 120 o The reaction was carried out at C for 6 h, and the reaction was monitored by TLC; after the reaction was complete, it was cooled to room temperature, the solvent was removed under reduced pressure, and purified by flash silica gel column chromatography (petroleum ether:ethyl acetate=10:1) to obtain the yellow solid product 1b, which was produced as The rate is 68%, and its structural formula is,
[0023] ;
[0024] Product characterization: 1 H NMR (400 MHz, CDCl 3 ) δ 7.94 (d, J = 8.1 Hz, 2H), 7.27 – 7.12(m, 7H), 7.12 – 7.00 (m, 3H), 6.97 – 6.89 (m, 2H), 5.60 (s, 2H), 2.32 (s,3H). 13 C NMR (100 MHz, CDCl 3 ) δ 151.5, 138.3, 134.7, 129.6, 129.5, 129.0, 128.9, 128.5, 128.0, 127.7, 127.5, 127.1, 127....
Embodiment 3
[0026] 1-Benzyl-5-(2-naphthyl)-4-(phenylselenyl)-1 H Synthesis of -1,2,3-triazole:
[0027] Add 0.5 mmol of 2-propynenaphthoic acid, 0.5 mmol of diphenyldiselenide, 0.5 mmol of benzyl azide, 10 mol% of copper chloride, 0.6 mmol of sodium carbonate and 3 mL of chloride to the sealed tube Benzene, at 120 o The reaction was carried out at C for 6 h, and the reaction was monitored by TLC; after the reaction was complete, it was cooled to room temperature, the solvent was removed under reduced pressure, and purified by flash silica gel column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain the yellow solid product 1c, which was produced The rate is 60%, and its structural formula is, ;
[0028] Product characterization: 1 H NMR (400 MHz, CDCl 3 ) δ 8.52 (s, 1H), 8.23 – 8.20 (m, 1H), 7.92 – 7.73 (m, 3H), 7.44 – 7.41 (m, 2H), 7.21 – 7.18 (m, 5H), 7.12 – 6.89(m , 5H), 5.65 (s, 2H). 13 C NMR (100 MHz, CDCl 3 ) δ 151.3, 134.7, 133.1, 129.6, 129.5, 129.3, 128...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com