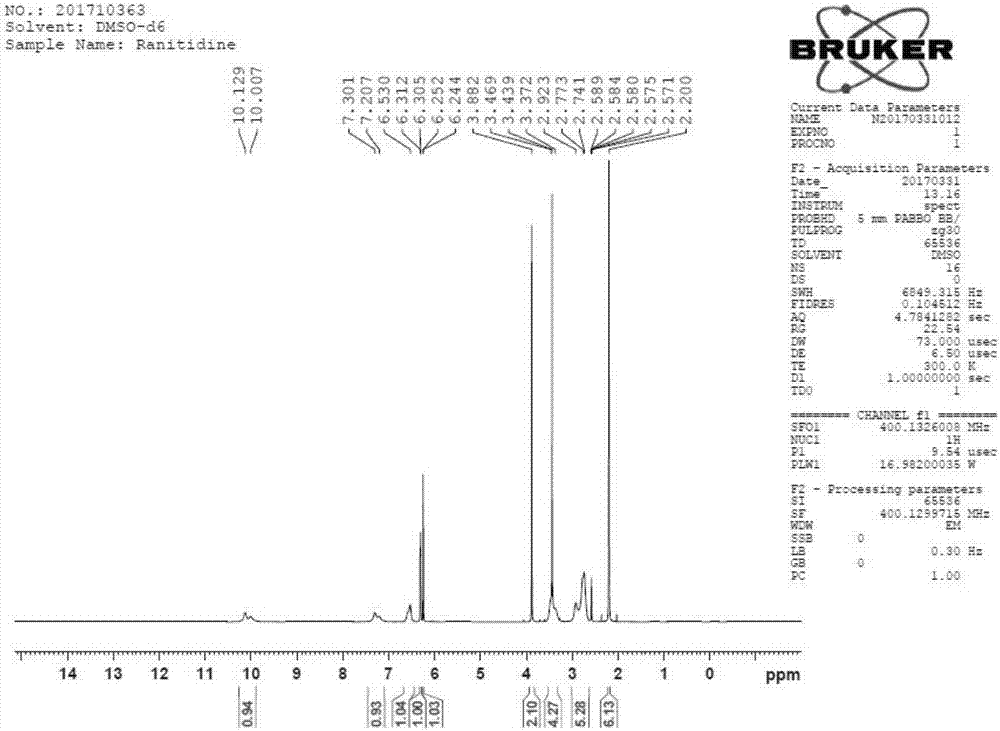
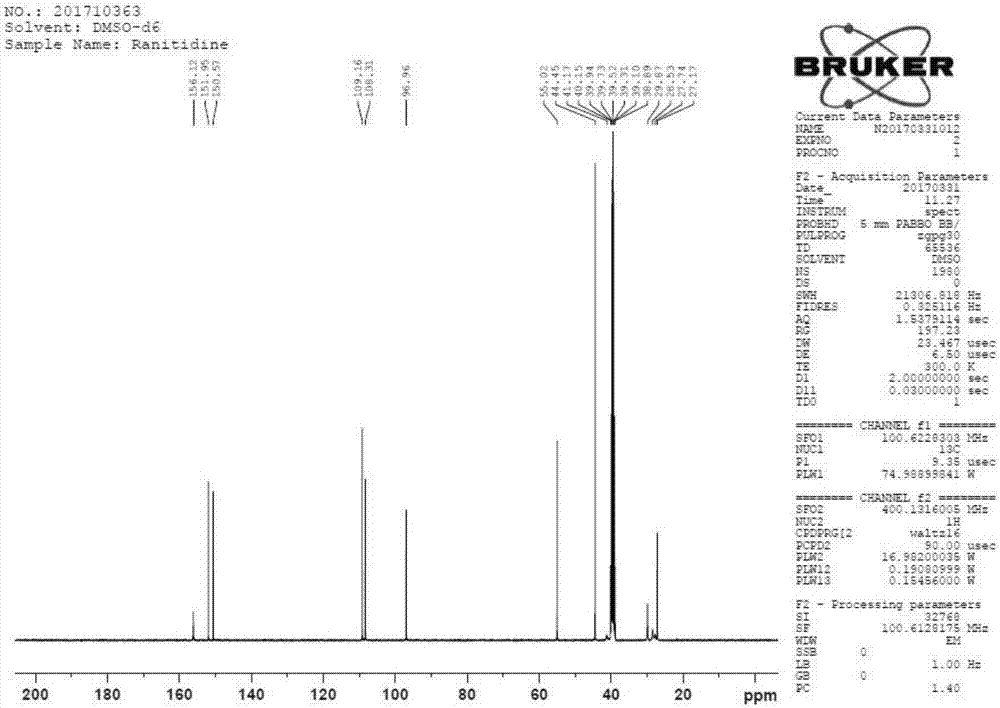
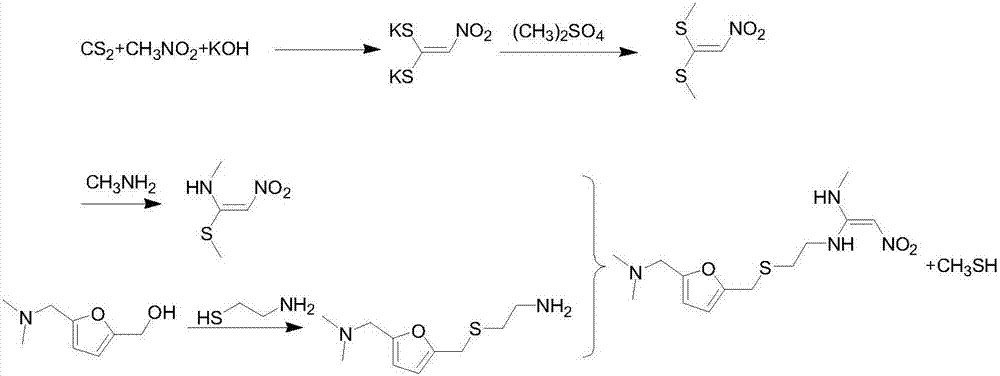
Method for synthesizing ranitidine
A technology of ranitidine and mixed acid is applied in the field of synthesizing ranitidine, which can solve the problems of serious environmental pollution and unsafe traditional processes, and achieve the effects of reducing potential safety hazards, facilitating industrial production and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of vinylidene chloride
[0035] Add 100g of 1,1,2-trichloroethane into a four-neck flask equipped with a distillation device, heat the feed liquid to 30-35°C, add 100g of 30% sodium hydroxide solution dropwise with a constant pressure dropping funnel, drop During the addition process, the liquid was continuously condensed and received by the distillation device to obtain 70 g of vinylidene chloride with a yield of 96% and a GC purity of >95%.
[0036] (2) Synthesis of 1,1-dichloro-2-nitroethylene
[0037] Add 80g of concentrated hydrochloric acid and 80g of concentrated nitric acid into the three-necked flask, add 60g of vinylidene chloride dropwise at a temperature of 20-25°C, continue to keep warm for 3 hours after dropping, pour into 100g of ice water after the reaction, add 100g of chloroform for extraction , collect the chloroform layer and wash twice with 5% sodium bicarbonate solution, dry the washed chloroform with anhydrous magnesium sulfate for ...
Embodiment 2
[0045] (1) Synthesis of vinylidene chloride
[0046] Add 100g of 1,1,2-trichloroethane into a four-neck flask equipped with a distillation device, heat the feed liquid to 30°C, and add 110g of 30% sodium hydroxide solution dropwise with a constant pressure dropping funnel. The liquid is continuously condensed and received by the distillation device to obtain vinylidene chloride.
[0047] (2) Synthesis of 1,1-dichloro-2-nitroethylene
[0048] Add 62g of concentrated hydrochloric acid and 68g of concentrated nitric acid into the three-necked flask, control the temperature and add 65g of vinylidene chloride dropwise at 20°C, continue the heat preservation reaction for 2 hours after dropping, pour into 100g of ice water after the reaction, add 100g of dichloromethane for extraction , the dichloromethane layer was collected and washed twice with 5% sodium bicarbonate solution, and the washed dichloromethane was dried with anhydrous magnesium sulfate for 1 hour, filtered, and conce...
Embodiment 3
[0056] (1) Synthesis of vinylidene chloride
[0057] Add 100g of 1,1,2-trichloroethane into a four-necked flask equipped with a distillation device, heat the feed liquid to 35°C, and add 100g of 30% sodium hydroxide solution dropwise with a constant pressure dropping funnel. The liquid is continuously condensed and received by the distillation device to obtain vinylidene chloride.
[0058] (2) Synthesis of 1,1-dichloro-2-nitroethylene
[0059] Add 82g of concentrated hydrochloric acid and 98g of concentrated nitric acid into the three-necked flask, add 60g of vinylidene chloride dropwise at a temperature of 20-25°C, and continue to keep warm for 4 hours. After the reaction, pour into 100g of ice water and extract with 100g of chloroform. , the chloroform layer was collected and washed twice with 5% sodium bicarbonate solution, and the washed chloroform was dried with anhydrous magnesium sulfate for 1 hour, filtered, and concentrated to obtain 1,1-dichloro-2-nitroethylene.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com