Polymer or micro-molecular material having molecular main chain comprising dibenzothiophene sulfoxide group, preparation method and application thereof
A technology of benzodisulfoxide phene and benzodisulfoxide, which is applied in the field of organic solar cells, can solve the problems of low carrier mobility, narrow absorption spectrum, poor stability, etc., achieve broad absorption spectrum and enhance molar absorption coefficient, the effect of improving coplanarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
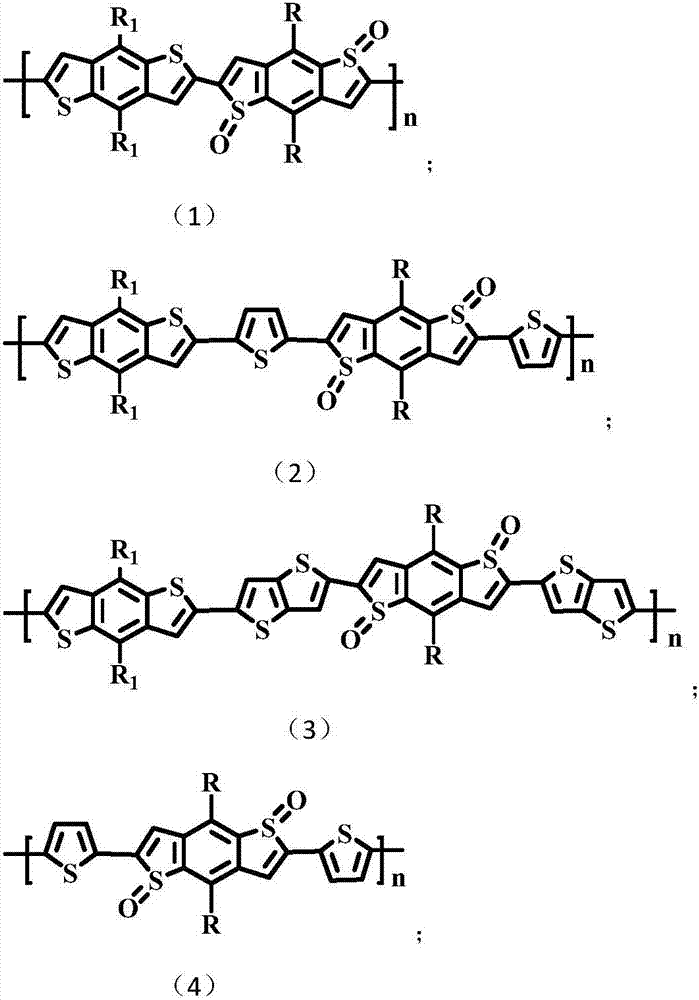
[0029] In the present embodiment, the polymer structural formula is as follows:
[0030]
[0031] The preparation of this polymer comprises the steps:
[0032] (1) Preparation of benzodisulfoxide phenyl acceptor unit
[0033]
[0034] Add 0.3g of compound 1 and 20mL of dichloromethane into a round-necked flask, place the flask in a low-temperature bath and cool it to 5°C, add dropwise 10mL of hydrogen peroxide and 15mL of formic acid with a concentration of 30%, and stir for 12 hours. After the reaction was completed, formic acid was removed by adding a saturated sodium bicarbonate solution dropwise, and the product was extracted with dichloromethane and dried with magnesium sulfate. After being spin-dried, it was passed through a silica gel column to obtain a light yellow product 2 with a mass of 0.16 g and a yield of 51%. 1 H-NMR (400MHz, CDCl 3 ):δ7.39(d,2H),6.71(d,2H),4.41(t,4H),2.0-1.81(m,4H),1.55-1.45(m,4H),1.39-1.33(m,8H ), 0.89(t,6H). The calculated molecula...
Embodiment 2
[0041] In the present embodiment, the polymer structural formula is as follows:
[0042]
[0043] The preparation of this polymer comprises the steps:
[0044]
[0045] Add 0.1g of compound 2, 0.064g of 2,5-trimethyltinthiophene and 10mL of toluene into a three-necked flask, replace nitrogen three times, add 4mg of catalyst tris(dibenzylideneacetone)dipalladium and 4.63mg of triphenylphosphine , and then replace nitrogen three times, and react under reflux for 24 hours. After the reaction, the product was subjected to Soxhlet extraction, using methanol, acetone and n-hexane as solvents, and finally using toluene to obtain 50 mg of the polymer product, with a yield of 57%.
[0046] 1 H-NMR (400MHz, CDCl 3 ):δ7.3-7.2(m,2H),7.0-6.8(m,2H),4.0-3.78(m,4H),2.0-1.9(m,2H),1.3-1.2(m,16H),1 -0.9(m,12H).
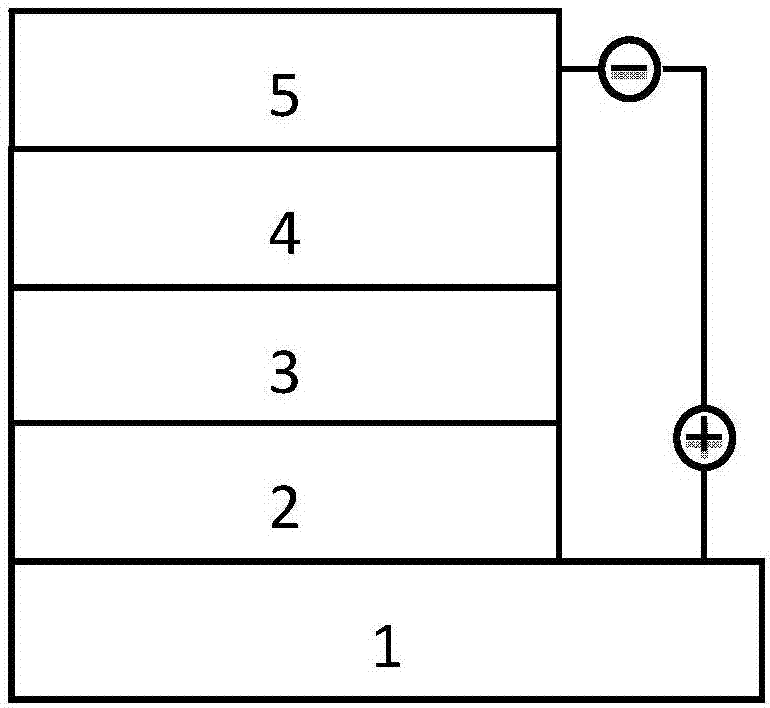
[0047] The above-prepared polymers can be used as donor materials in organic solar cells. The structure of the organic solar cell is as figure 1 As shown, the anode is made...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com