Rigid porous framework polymer with gas adsorption performance, preparation method of polymer and application
A technology of porous polymers and rigid skeletons, applied in separation methods, alkali metal compounds, chemical instruments and methods, etc., can solve the problems of strong corrosion, easy to be volatile, easy to decompose, and difficult to regenerate, so as to improve adsorption efficiency and increase The effect of contact area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
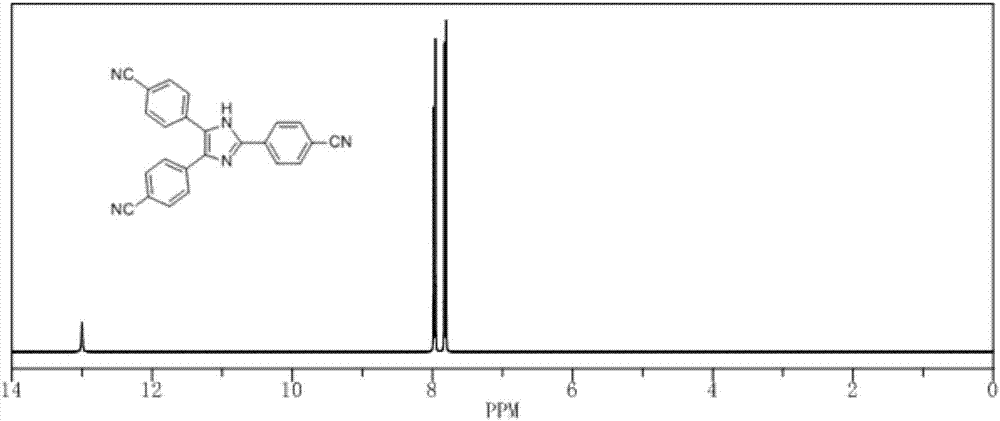
[0080] The synthesis of 2,4,5-tris(4-cyanophenyl)-1-hydroimidazoles (CMPs) monomers was carried out in an autoclave.
[0081] 1 molar part of 2,4,5-tris(4-bromophenyl)-1-hydroimidazole (0.533g), 3 molar parts of cuprous cyanide (0.268g), 1 molar equivalent of sodium carbonate (0.105g ) and refined 80 mole parts of DMF were added into the autoclave. Reaction at 150°C for 12h gave a dark purple solution. After cooling down to room temperature, filter with suction. Add concentrated ammonia water to the filtrate until the solution turns into a dark blue system. The solution was gradually added dropwise to an ice-water solution with a pH value of 9, stirred overnight and suction-filtered. The filter cake was dried and separated by column chromatography (dichloromethane:n-hexane, 1:3) to obtain a light yellow powdery solid (0.259g).
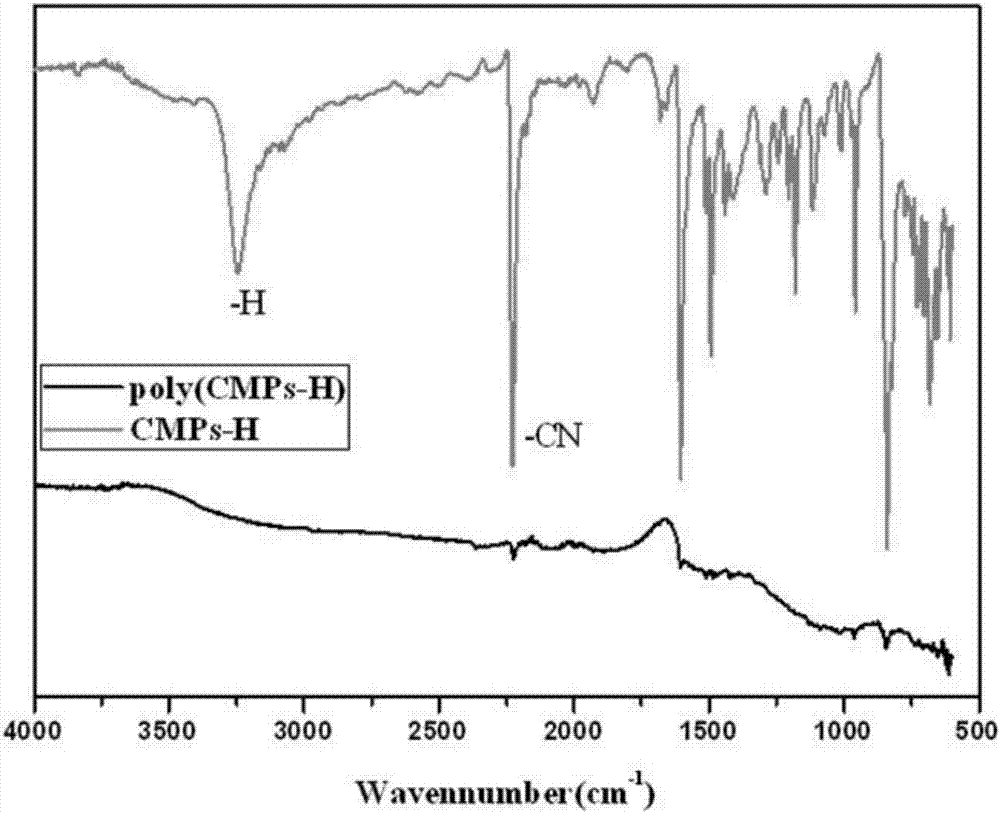
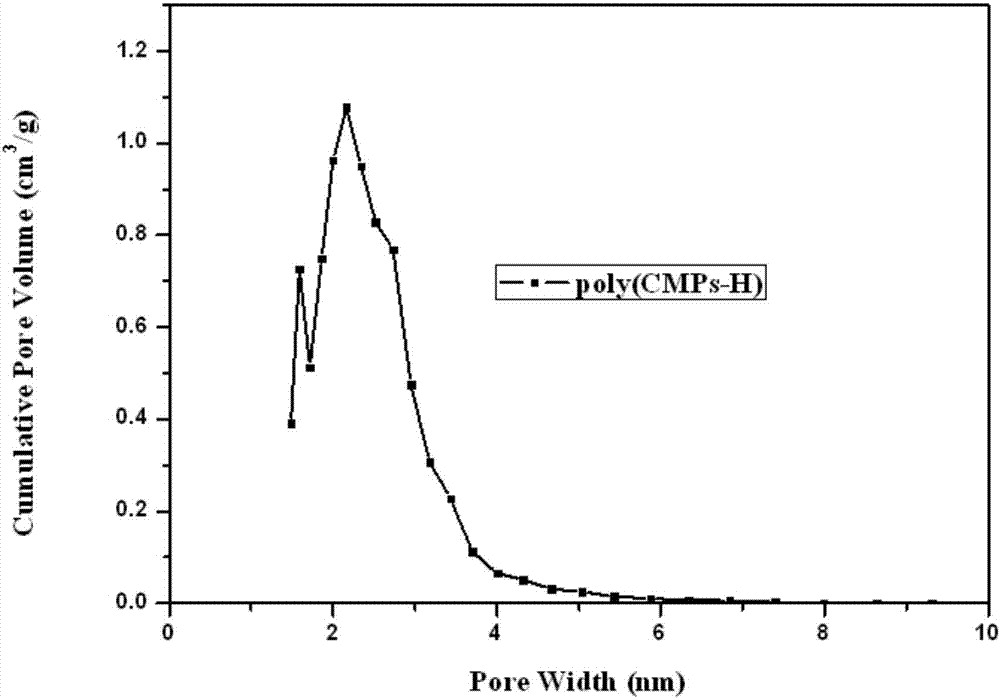
[0082] The obtained (CMPs) monomer 2,4,5-tris(4-cyanophenyl)-1-hydroimidazole (0.371g) and zinc chloride (0.408g) were mixed in a mortar and morta...
Embodiment 2
[0085] The synthesis of 2,4,5-tris(4-cyanophenyl)-1-hydroimidazoles (CMPs) monomers was carried out in an autoclave.
[0086] 1 molar part of 2,4,5-tris(4-bromophenyl)-1-hydroimidazole (0.533g), 3 molar parts of cuprous cyanide (0.268g), 1 molar equivalent of sodium carbonate (0.105g ) and refined 80 mole parts of DMF were added into the autoclave. Reaction at 150°C for 12h gave a dark purple solution. After cooling down to room temperature, filter with suction. Add concentrated ammonia water to the filtrate until the solution turns into a dark blue system. The solution was gradually added dropwise to an ice-water solution with a pH value of 9, stirred overnight and suction-filtered. The filter cake was dried and separated by column chromatography (dichloromethane:n-hexane, 1:3) to obtain a light yellow powdery solid (0.259g).
[0087] The obtained (CMPs) monomer 2,4,5-tris(4-cyanophenyl)-1-hydroimidazole (0.371g) and zinc chloride (0.408g) were mixed in a mortar and morta...
Embodiment 3
[0089] 1 molar part of 2,4,5-tris(4-bromophenyl)-1-methylimidazole (0.547g), 5 molar parts of cuprous cyanide (0.447g), 2 molar equivalents of sodium carbonate (0.210 g) and refined 90 mole parts of DMF were added to the autoclave. Reaction at 160°C for 10 h gave a dark purple solution. After cooling down to room temperature, filter with suction. Add concentrated ammonia water to the filtrate until the solution turns into a dark blue system. The solution was gradually added dropwise to an ice solution with a pH value of 8, stirred overnight and suction filtered. The filter cake was dried and separated by column chromatography (dichloromethane: n-hexane, 1:3) to obtain a light yellow powdery solid.
[0090] Zinc chloride is ground first, and then the obtained (CMPs) monomer 2,4,5-tris(4-cyanophenyl)-1-methylimidazole and zinc chloride are grinded at a molar ratio of 1:5 Grind in a bowl until powdered, and dry at 150°C overnight to remove water. The mixture was slowly added...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com