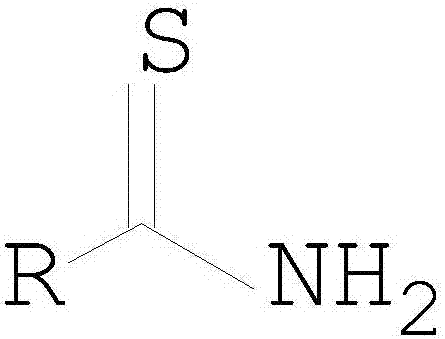
Process method for synthesizing thioamide
A technology for synthesizing thioamides and thioamides, applied in organic chemistry and other directions, can solve the problems of difficult industrialization, phosphorus waste water, expensive reagents, etc., and achieve the effects of improved effective utilization, mild reaction conditions, and enhanced safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
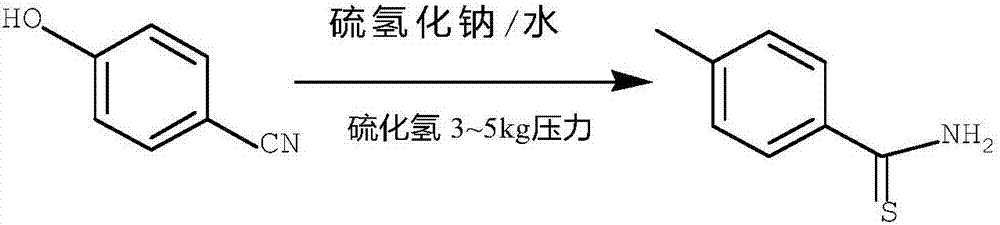
[0044] Add 11g of sodium hydrosulfide, 6g of p-hydroxybenzonitrile, 6.9mL of triethylamine, and 40mL of ethanol into a 100mL three-necked flask, control the temperature in an ice bath to 5-10°C, add 27.5g of triethylamine hydrochloride solid in batches, add After raising the temperature to 50° C., keeping the temperature under stirring for 12 hours, TLC monitored the reaction to be complete, and liquid phase monitoring showed that the conversion rate was above 95%, and the final yield was 92%. After the reaction, the ethanol recovered by distillation can be used directly, adding 40 mL of ethanol to the residue of distillation to make a slurry at 50°C, stirring for 0.5 hours, and filtering to obtain the by-product sodium chloride solid salt with higher purity, which can be sold for processing. The ethanol filtrate was concentrated to dryness, and the residue was slurried by adding 25 mL of water, filtered, and dried to obtain 7.32 g of yellowish solid. The liquid phase content i...
Embodiment 2
[0046] Add 16.8g of sodium hydrosulfide, 6g of p-hydroxybenzonitrile, 1.7mL of dimethylamine, and 40mL of methanol into a 100mL three-necked flask, control the temperature in an ice bath at -5~0℃, add 28.7g of isopropylamine hydrochloride solid in batches, After the addition, the temperature was raised to 30°C, and the temperature was kept under stirring for 11 hours. TLC monitored the reaction to be complete, and liquid phase monitoring showed that the conversion rate was above 95%, and the final yield was 92%. After the reaction, the methanol recovered by distillation can be directly used, and the residue of distillation is added with 40mL methanol to make a slurry at 50°C, stirred for 0.5 hours, and filtered to obtain the by-product sodium chloride solid salt with higher purity, which can be sold for processing. The methanol filtrate was concentrated to dryness, and the residue was slurried by adding 25 mL of water, filtered, and dried to obtain 6.7 g of ochre solid. The liq...
Embodiment 3
[0048] Add 31.2g of sodium sulfide, 6g of p-hydroxybenzonitrile, 12.8mL of isopropylamine, and 50mL of isopropanol into a 100mL three-necked flask, control the temperature in an ice bath at -2~2℃, and add 42g of dimethylamine acetate solid in batches. After adding, raise the temperature to 10°C, keep it for 10 hours under stirring, and filter with suction to obtain the by-product sodium acetate solid salt with higher purity, which can be sold for processing. The filtrate was distilled to recover the isopropanol and used directly in the next batch, the distillation residue was added with an appropriate amount of isopropyl ether to make a slurry, filtered with suction, and dried to obtain 6.7 g of an earthy yellow solid with a liquid content of 93% and a yield of 81.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com