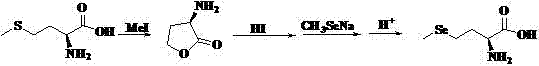
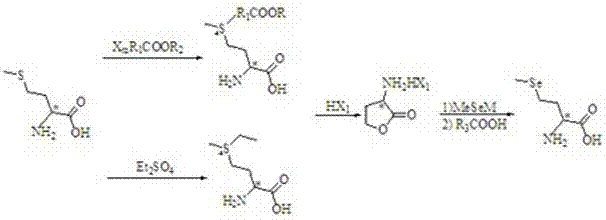
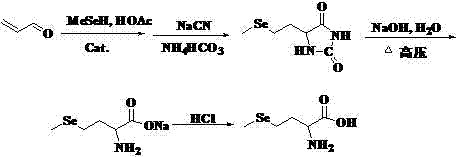
Preparation method of high-optical-purity D- or L-selenomethionine
A technology of selenomethionine and optical purity, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of unproduct chemical purity and optical purity reporting, inability to meet environmental protection requirements, thiomethyl ether odor, etc., and achieve steps that are easy to operate, The effect of simple steps and simple reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Step (1): Under the protection of nitrogen, add 153.30g L-methionine, 250g water, 126.21g methyl chloroformate, 300g acetic acid, and 300mL ethanol into a 1L four-necked flask in sequence. After stirring at room temperature, the temperature is raised to 90°C. After the reaction is complete, the solvent is evaporated.
[0051] Step (2): At low temperature, add 400g of 1,4-dioxane solution with 12% HCl content to the above system, then heat it to reflux for 10h, then cool to room temperature, stir for 2h, vacuum filter, filter The cake is washed with 1,4-dioxane, and the white solid obtained is L-α-amino-γ-butyrolactone hydrochloride, the yield is 79.92%, and the chemical purity is> 99%, optical purity ee> 99%.
[0052] Step (3): Under the protection of nitrogen, mix 13.75 g α-amino-γ-butyrolactone hydrochloride and 40 mL DMF, and stir evenly at room temperature. Dissolve 15.2 g of sodium methyl selenoxide in 20 mL of DMF, add it to the above system, and reflux for 2 hours. ...
Embodiment 2
[0054] Step (1): Under the protection of nitrogen, add 153.10g D-methionine, 420g water, 200mL methanol, and 158.21g diethyl sulfate into a 2L four-necked flask in sequence. After stirring at room temperature, slowly add 45g concentrated sulfuric acid dropwise. After the addition, the temperature was raised to 30-40°C, and after 10 hours, methanol was recovered under reduced pressure. Then adjust the pH of the reaction solution to 8-9 with anhydrous sodium carbonate solid, and continue to react at 40°C for 10 hours. After the reaction is over, the solvent is evaporated under reduced pressure.
[0055] Step (2): Add 260 mL 37% concentrated hydrochloric acid solution to the above system, heat to reflux for 4 hours, evaporate the solvent under reduced pressure to obtain a solid or viscous substance, use hot ethanol to extract the product, and the crude product is subjected to conventional recrystallization treatment Get α-
[0056] Amino-γ-butyrolactone hydrochloride, the yield is 6...
Embodiment 3
[0059] The difference between this embodiment and the first embodiment is that the raw materials used in step (1) are bromoformic acid, chloroformic acid, chloroacetic acid, bromoacetic acid, 1-chloropropionic acid, 1-chlorobutyric acid, methyl chloroacetate, Ethyl chloroformate, ethyl chloroformate, etc. The results are shown in the table below.
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com