Preparation method and application of bismuth metal auto-doping halogenated bismuth oxide
A bismuth oxyhalide, self-doping technology, applied in chemical instruments and methods, bismuth compounds, inorganic chemistry, etc., to achieve the effects of high detection sensitivity, low cost consumption, and obvious practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
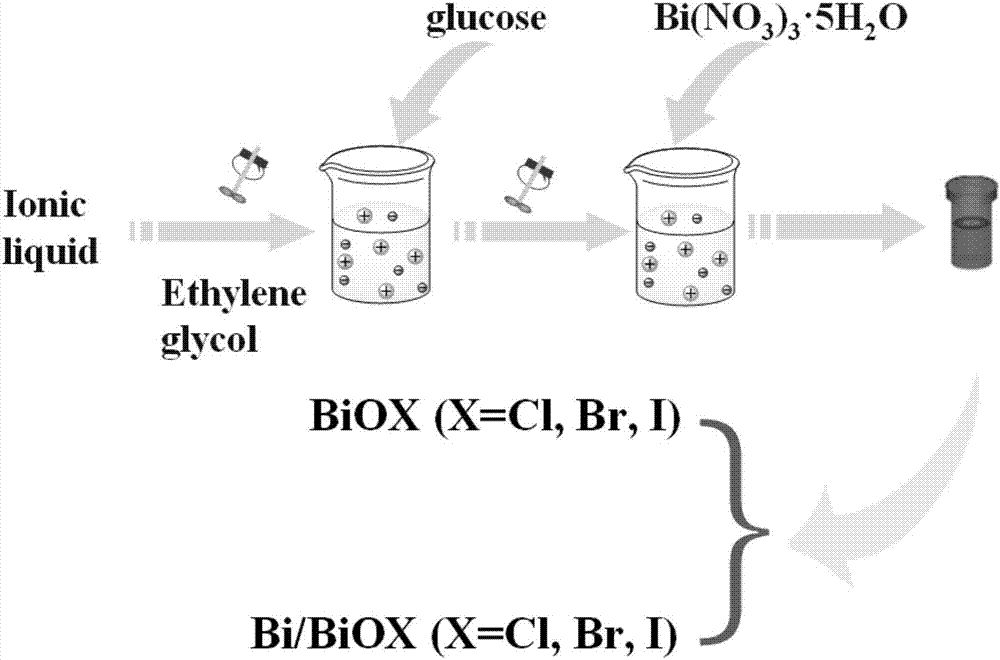
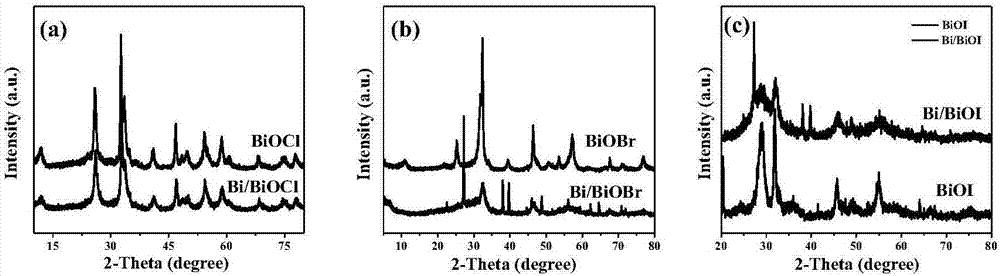
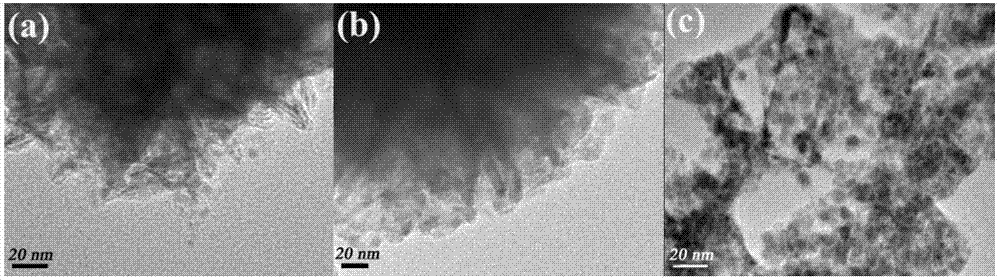
[0036] (1) The preparation of bismuth oxychloride monomer material is that solvothermal reaction one-step method makes: at first, take the 1-hexadecyl-3-methylimidazolium chloride salt of 1mmol ([C 16mim]Cl) ionic liquid into a 25mL polytetrafluoroethylene-lined reactor, secondly, pipette 20mL of ethylene glycol solution into the kettle, and fully dissolved. Add 1 mmol of bismuth nitrate pentahydrate into the kettle, and keep stirring for half an hour until the mixture is uniform. After reacting at 160°C for 24h, it was naturally cooled to room temperature; the final product was washed with alcohol, washed with water, centrifuged, and dried at 50°C for 12h to obtain BiOCl.
[0037] (2) The preparation of bismuth oxychloride material self-doped with bismuth metal is to reduce bismuth nitrate pentahydrate to obtain by glucose: first, weigh 1mmol of 1-hexadecyl-3-methylimidazolium chloride salt ( [C 16 mim]Cl) ionic liquid into a 25mL polytetrafluoroethylene-lined reactor, seco...
Embodiment 2
[0042] (1) The preparation of bismuth oxybromide monomer material is that solvothermal reaction one-step method makes: at first, take the 1-hexadecyl-3-methylimidazolium bromide ([C 16 mim] Br) of the ionic liquid into a 25mL polytetrafluoroethylene-lined reactor, and secondly, pipette 20mL of ethylene glycol solution into the kettle, and fully dissolved. Add 1 mmol of bismuth nitrate pentahydrate into the kettle, and keep stirring for half an hour until the mixture is uniform. After reacting at 140°C for 24h, it was naturally cooled to room temperature; the final product was washed with alcohol, washed with water, centrifuged, and dried at 50°C for 12h to obtain BiOBr.
[0043] (2) The preparation of bismuth oxybromide material self-doped with bismuth metal is to reduce bismuth nitrate pentahydrate to obtain by glucose: first, weigh 1mmol of 1-hexadecyl-3-methylimidazolium bromide ( [C 16 mim] Br) of the ionic liquid into a 25mL polytetrafluoroethylene-lined reactor, and se...
Embodiment 3
[0048] (1) The preparation of bismuth oxyiodide monomer material is obtained by solvothermal reaction one-step method: first, the ionic liquid of 1-butyl-3-methylimidazolium iodide salt ([Bmim]I) that takes 1mmol is put into 25mL In a polytetrafluoroethylene-lined reaction kettle, secondly, pipette 20mL of ethylene glycol solution into the kettle and fully dissolve it. Add 1 mmol of bismuth nitrate pentahydrate into the kettle, and keep stirring for half an hour until the mixture is uniform. After reacting at 160°C for 24h, it was naturally cooled to room temperature; the final product was washed with alcohol, washed with water, centrifuged, and dried at 50°C for 12h to obtain BiOI.
[0049] (2) Bismuth metal self-doped bismuth oxyiodide material is prepared by reducing bismuth nitrate pentahydrate by glucose: first, weigh 1 mmol of 1-butyl-3-methylimidazolium iodide ([Bmim ] The ionic liquid of I) is put into 25mL polytetrafluoroethylene-lined reactor, secondly, pipette 20mL...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com