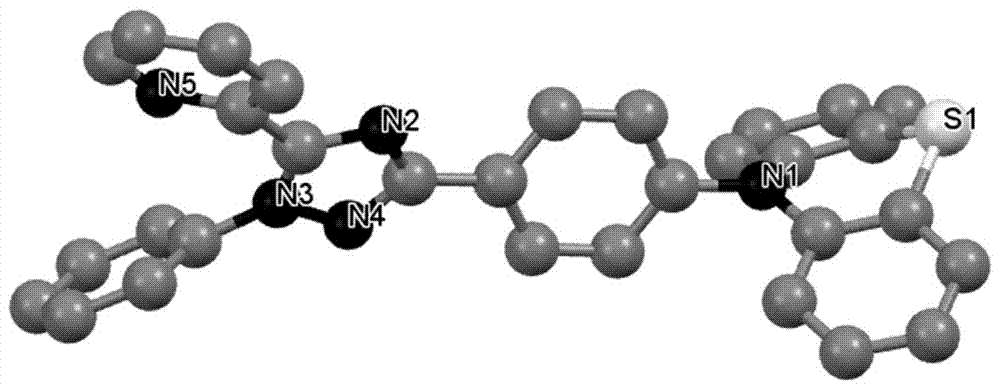
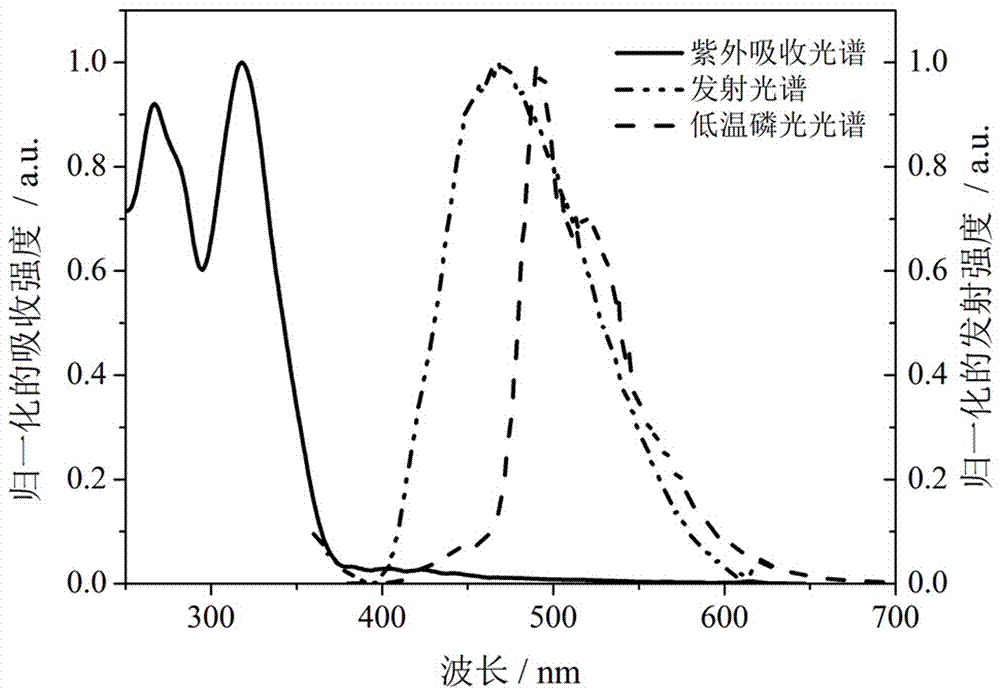
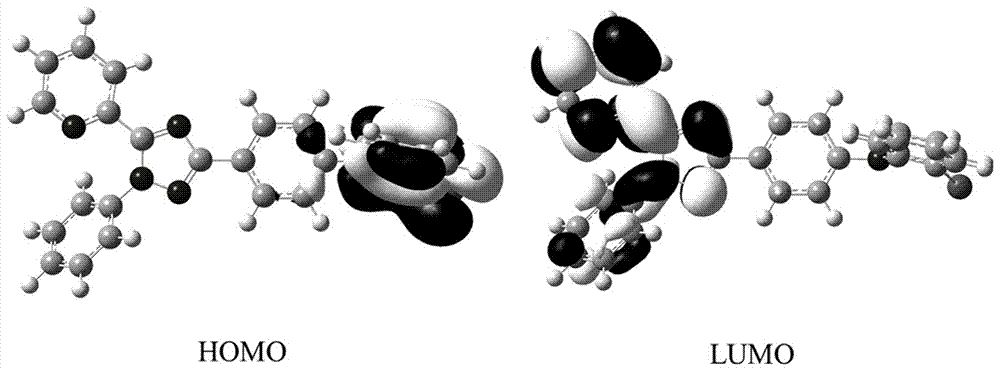1,2,4-Triazole acceptor-based thermally activated delayed fluorescence material
A technology of thermally activated delay and fluorescent materials, which is applied in the field of organic photoelectric functional materials, can solve the problems of insufficient electron-absorbing ability of acceptor materials, and the emission spectrum is not in the deep blue region, so as to improve the RISC transition probability, improve luminous efficiency, and increase The effect of steric hindrance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Take 5.21g (50mmol) of 2-cyanopyridine, 2.50g (50mmol) of hydrazine hydrate, and 25mL of ethanol, and add them to a 250mL two-neck round-bottomed flask in turn, and react at a low temperature of 0°C for 8h to produce a viscous light yellow paste, at room temperature The excess ethanol was removed under vacuum, the solid was washed with a small amount of ether, filtered, and dried under vacuum at room temperature for 3 hours to obtain white crystals of (2-pyridine)aminohydrazone.
[0048] Weigh 4.08g (30mmol) of (2-pyridine)aminohydrazone into a 250mL two-neck round bottom flask, and then add Na in turn 2 CO 3 3.15g (30mmol), 7.00g (30mmol) of 4-bromobenzoyl chloride, 30mL of tetrahydrofuran, react at room temperature for 6h, and filter. The filtrate was refluxed in 30ml of ethylene glycol for 30min, dehydrated and closed, filtered, dried in vacuum for 8h, and recrystallized with ethanol to obtain 2-(3-(4-bromophenyl)-1H-1,2,4-tri Azazol-5-yl)pyridine is white needle-like c...
Embodiment 2
[0064] The 2-(1-phenyl-3-(4-bromophenyl)-1H-1,2,4-triazol-5-yl)pyridine (1.88g, 5mmol) prepared in Example 1 and phenoxa The oxazine (1.28g, 7mmol) was added to a 100mL two-necked round bottom flask filled with nitrogen, and then tris(dibenzylideneacetone)dipalladium (270mg, 0.3mmol), tri-tert-butylphosphine tetrafluoroborate (340mg, 0.3mmol), sodium tert-butoxide (960mg, 10mmol) and 50mL toluene, heated to reflux temperature for 24h under nitrogen atmosphere. The reaction solution was cooled to room temperature, 50 mL of water was added, and extraction was performed with 200 mL of dichloromethane. The extract was dried over anhydrous magnesium sulfate and purified by petroleum ether / dichloromethane (2:1) column chromatography to obtain 10-(4-( 1-Phenyl-5-(pyridin-2-yl)-1H-1,2,4-triazol-3-yl)phenyl)-10H-phenoxazine (TAZ-PXZ) white solid, yield 59%.
[0065] The structural formula of the prepared TAZ-PXZ is as follows.
[0066]
[0067] 1 H NMR (600 MHz, CDCl 3 , δ): 8.56-8.51 (m...
Embodiment 3
[0073] Take 2-(1-phenyl-3-(4-bromophenyl)-1H-1,2,4-triazol-5-yl)pyridine (1.88g, 5mmol) prepared in Example 1 and 9, 9-Dimethylacridine (1.67g, 8mmol) was added to a 100mL two-necked round bottom flask filled with nitrogen, then tris(dibenzylideneacetone)dipalladium (270mg, 0.3mmol), tri-tert-butyl tetrafluoroborate Phosphine (340 mg, 0.3 mmol), sodium tert-butoxide (960 mg, 10 mmol) and 50 mL of toluene were heated to reflux temperature for 24 hours under a nitrogen atmosphere. The reaction solution was cooled to room temperature, 50 mL of water was added, and extraction was performed with 200 mL of dichloromethane. The extract was dried over anhydrous magnesium sulfate and purified by petroleum ether / dichloromethane (3:1) column chromatography to obtain 9,9-dimethyl Base-10-(4-(1-phenyl-5-(pyridin-2-yl)-1H-1,2,4-triazol-3-yl)phenyl)-9,10-dihydroacridine Pyridine (TAZ-DMAC) is a white solid with a yield of 61%.
[0074] The structural formula of the prepared TAZ-DMAC is as fol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com