Preparation method of bendroflumethiazide tablet
A Benfluflumethiazide tablet and Benfluflumethiazide technology, which is applied in the field of preparation of Benfluflumethiazide Tablets, can solve problems such as exceeding the standard, large substances, and content differences between Benfluflumethiazide tablets, and achieve high efficiency, Avoid high temperature problems, the effect of rapid drug dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
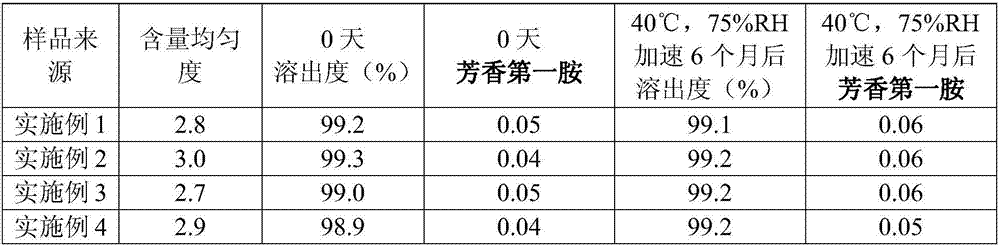
Embodiment 1
[0026] The present embodiment provides a preparation method of bendroflumethiazide tablet, comprising the following steps:
[0027] (1) Add bendroflumethiazide and microcrystalline cellulose PH-102 with water and mix uniformly to obtain suspension 1;
[0028] (2) Suspension 1 is wet pulverized to obtain Suspension 2; said wet pulverization adopts the mode of colloid mill and high-speed stirring bead mill to carry out, and wet grinding adopts low-temperature water circulation cooling to reduce the temperature of grinding, circulating water The temperature is less than 15°C, and the particle size D90 of bendroflumethiazide after wet grinding is less than 30 μm;
[0029] (3) Add the suspension 2 into lactose and cornstarch by spraying, and use wet granulation;
[0030] (4) Dry the granules, pass through a 30-mesh sieve, add sodium starch glycolate and magnesium stearate, and compress into tablets. The batch size is 100,000 tablets, and the specification is 2.5 mg / tablet.
[003...
Embodiment 2
[0033] The present embodiment provides a preparation method of bendroflumethiazide tablet, comprising the following steps:
[0034] (1) Add bendroflumethiazide and microcrystalline cellulose PH-102 with water and mix uniformly to obtain suspension 1;
[0035] (2) Suspension 1 is wet pulverized to obtain Suspension 2; said wet pulverization adopts the mode of colloid mill and high-speed stirring bead mill to carry out, and wet grinding adopts low-temperature water circulation cooling to reduce the temperature of grinding, circulating water The temperature is less than 15°C, and the particle size D90 of bendroflumethiazide after wet grinding is less than 30 μm;
[0036] (3) Add the suspension 2 into lactose and cornstarch by spraying, and use wet granulation;
[0037] (4) Dry the granules, pass through a 30-mesh sieve, add sodium starch glycolate and magnesium stearate, and compress into tablets. The batch size is 100,000 tablets, and the specification is 2.5 mg / tablet.
[003...
Embodiment 3
[0040] The present embodiment provides a preparation method of bendroflumethiazide tablet, comprising the following steps:
[0041] (1) Add bendroflumethiazide and microcrystalline cellulose PH-102 with water and mix uniformly to obtain suspension 1;
[0042] (2) Suspension 1 is wet pulverized to obtain Suspension 2; said wet pulverization adopts the mode of colloid mill and high-speed stirring bead mill to carry out, and wet grinding adopts low-temperature water circulation cooling to reduce the temperature of grinding, circulating water The temperature is less than 15°C, and the particle size D90 of bendroflumethiazide after wet grinding is less than 30 μm;
[0043] (3) Add the suspension 2 into lactose and cornstarch by spraying, and use wet granulation;
[0044] (4) Dry the granules, pass through a 30-mesh sieve, add sodium starch glycolate and magnesium stearate, and compress into tablets. The batch size is 100,000 tablets, and the specification is 2.5 mg / tablet.
[004...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com