Preparation method of catalyst for isobutylene preparation through oxidative dehydrogenation of isobutane
A technology for oxidative dehydrogenation and production of isobutene, which is applied in the direction of catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, carbon compound catalyst, etc. Catalyst cost and other issues, to achieve the effect of facilitating industrial scale-up, reducing concentration difference, and reducing metal consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
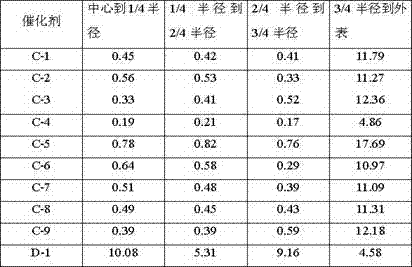
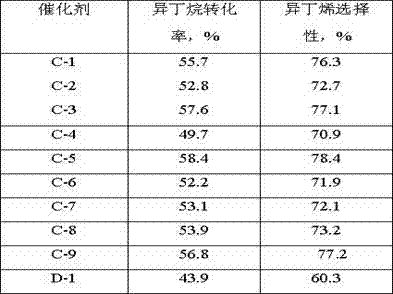
[0028] Weigh 2.97g of nickel nitrate and 0.55g of ammonium tungstate and dissolve them in 14mL of deionized water to obtain solution A; use equal volume impregnation method to load on 16g of alumina carrier (pore volume is 0.73mL / g, specific surface area is 253m 2 / g, strip shape, equivalent diameter 1.5mm), impregnated at room temperature for 2h, aged for 4h, dried at 80°C for 12h, and calcined at 700°C for 4h to prepare catalyst precursor B, wherein the supported Ni was calculated as the final catalyst The supported W is 2% of the final catalyst based on the weight of the element; the catalyst precursor B is activated in a mixed atmosphere containing hydrogen, and the hydrogen volume content in the mixed gas is 80%, and the reducing condition is 450 ° C, 0.2 MPa (absolute pressure), reduction time 4h; Dissolve 0.74g ammonium molybdate in 15mL deionized water to obtain solution C, and mix it with 4 times the mass of furfural aqueous solution with a mass fraction of 40%, and th...
Embodiment 2
[0030] Weigh 2.97g of nickel nitrate and 0.55g of ammonium tungstate and dissolve them in 14mL of deionized water to obtain solution A; use equal volume impregnation method to load on 16g of silica carrier (pore volume is 0.97mL / g, specific surface area is 372m 2 / g, spherical shape, equivalent diameter 0.5mm), impregnated at room temperature for 2h, aged for 4h, dried at 80°C for 12h, and calcined at 700°C for 4h to obtain the catalyst precursor B, wherein, the supported Ni is calculated by element weight, which is the weight of the final catalyst 3%, the supported W is 2% of the final catalyst based on the weight of the element; the catalyst precursor B is activated in a mixed atmosphere containing hydrogen, the volume content of hydrogen in the mixed gas is 80%, and the reducing conditions are 450 ° C, 0.2 MPa ( Absolute pressure), the reduction time is 4h; Dissolve 0.74g ammonium molybdate in 15mL deionized water to obtain solution C, and mix it with 4 times the mass of fur...
Embodiment 3
[0032] Weigh 2.97g of nickel nitrate and 0.55g of ammonium tungstate and dissolve them in 14mL of deionized water to obtain solution A; use equal volume impregnation method to load on 16g of SBA-15 carrier (pore volume is 1.23mL / g, specific surface area is 701m 2 / g, strip shape, equivalent diameter 1.5mm), impregnated at room temperature for 2h, aged for 4h, dried at 80°C for 12h, and calcined at 700°C for 4h to prepare catalyst precursor B, wherein the supported Ni was calculated as the final catalyst The supported W is 2% of the final catalyst based on the weight of the element; the catalyst precursor B is activated in a mixed atmosphere containing hydrogen, and the hydrogen volume content in the mixed gas is 80%, and the reducing condition is 450 ° C, 0.2 MPa (absolute pressure), reduction time 4h; Dissolve 0.74g ammonium molybdate in 15mL deionized water to obtain solution C, and mix it with 4 times the mass of furfural aqueous solution with a mass fraction of 40%, and the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com