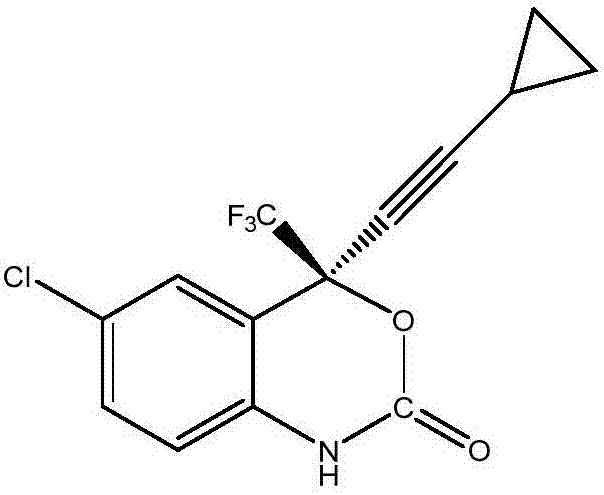
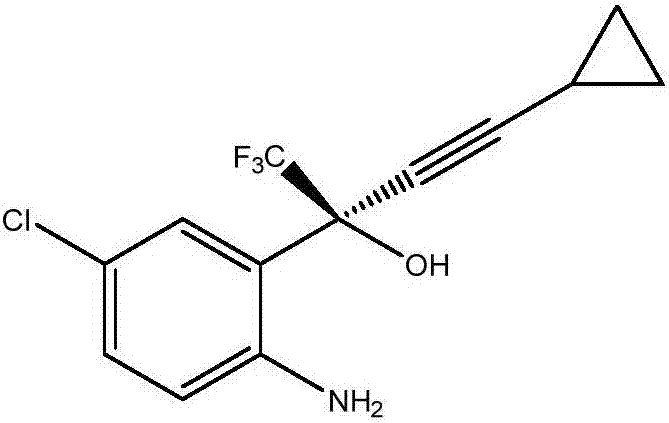
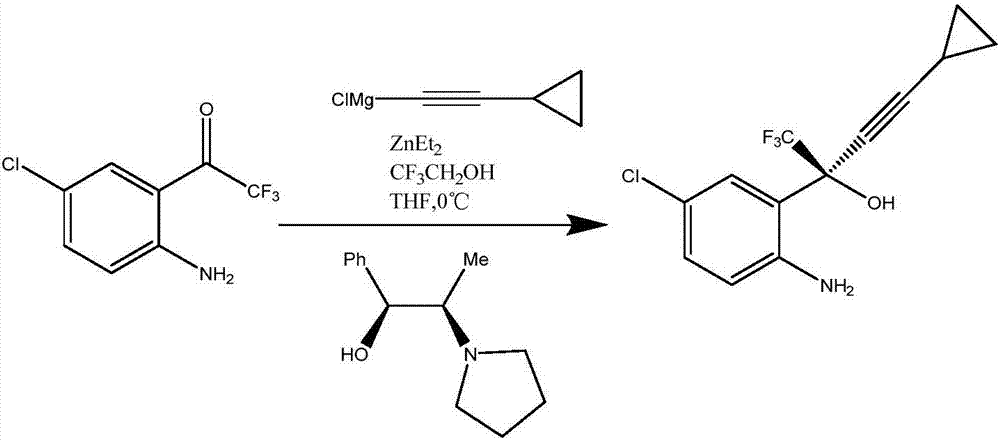
Efavirenz intermediate synthesizing method
A technology of efavirenz and an intermediate, which is applied in the field of organic chemical catalysis, can solve the problems of unfavorable industrial production, easy to absorb water again, high price and the like, and achieves the effects of easy recovery, beneficial to recovery and application, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 3.2g of magnesium chips and 10mL of 2-methyltetrahydrofuran into a round-bottomed flask, add bromoethane to initiate the reaction, control the temperature at 40°C, and add dropwise a mixture of 17.8g of bromobutane and 40mL of 2-methyltetrahydrofuran liquid. After the addition is complete, keep the reaction at 40°C for 3 hours, and then lower the temperature to below 10°C. Slowly add 8.5 g of cyclopropyneacetylene dropwise. After the addition, keep stirring at 10-15° C. for 2 hours, and place the prepared cyclopropyne-magnesium bromide format solution for later use. 32.4 g of potassium hydride was put into another round bottom flask, and 30 mL of 2-methyltetrahydrofuran was added. After the addition, the temperature was lowered to 10°C. Add neopentyl alcohol (8.8g), toluene (20g) and (1R,2S)-1-phenyl-2-(1-pyrrolidinyl)-1-propanol (7g) under control within 20°C and mix Liquid, mixed evenly for later use, and stirred at 10-15°C for 2 hours after adding. Then the t...
Embodiment 2
[0033]Add 3.84g of magnesium chips and 10mL of 2-methyltetrahydrofuran into a round-bottomed flask, add bromoethane to initiate the reaction, control the temperature at 40°C, and dropwise add a mixture of 21g of bromobutane and 40mL of 2-methyltetrahydrofuran . After the addition is complete, keep the reaction at 43°C for 2 hours, and then lower the temperature to below 10°C. Slowly add 10.2 g of cyclopropyneacetylene dropwise. After the addition is complete, keep stirring at 15° C. for 2 hours. The prepared cyclopropynemagnesium bromide format solution is set aside for later use. Put 24g of sodium hydride into another round bottom flask, and add 30mL of 2-methyltetrahydrofuran. After the addition, the temperature was lowered to 10°C. Add neopentyl alcohol (10.73g), toluene (20g) and (1R,2S)-1-phenyl-2-(1-pyrrolidinyl)-1-propanol (8g) under control within 20°C and mix Liquid, mixed evenly for later use, and stirred at 14°C for 2 hours after the addition was complete. Then ...
Embodiment 3
[0035] Add 5.96g of magnesium chips and 10mL of 2-methyltetrahydrofuran into a round-bottomed flask, add bromoethane to initiate the reaction, control the temperature at 41°C, add dropwise a mixture of 34.1g of bromobutane and 50mL of 2-methyltetrahydrofuran liquid. After the addition is complete, keep the reaction at 42°C for 2 hours, and then lower the temperature to below 5°C. Slowly add 15.9 g of cyclopropyneacetylene dropwise. After the addition, keep stirring at 15° C. for 2 hours, and place the prepared cyclopropynemagnesium bromide format solution for later use. 36.7 g of sodium hydride was put into another round bottom flask, and 60 mL of 2-methyltetrahydrofuran was added. After the addition, the temperature was lowered to 10°C. Add neopentyl alcohol (16.4g), toluene (20g) and (1R,2S)-1-phenyl-2-(1-pyrrolidinyl)-1-propanol (13.1g) within 20°C The mixed liquid was mixed evenly for later use, and stirred at 13° C. for 2 hours after the addition was completed. Then t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com