Macropore-mesopore ordered magnesium-aluminum composite oxide material and preparation method thereof
A technology of mesoporous ordering and aluminum compounding, which is applied in the preparation of alumina/hydroxide, preparation of liquid hydrocarbon mixtures, aluminum compounds, etc., can solve the problems of collapse of alumina mesoporous skeleton structure, few catalytic active sites, poor Alkali catalytic conversion activity and other issues, to achieve high catalytic activity, process is simple and easy, high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Under strong stirring at 30°C, add 0.4g citric acid and 3.2g EO to 20mL absolute ethanol solution containing 1.5g 6M nitric acid 106 PO 70 EO 106 , 0.51g of magnesium nitrate hexahydrate and 3.27g of aluminum isopropoxide were completely dissolved to obtain a clear solution; the resulting clear solution was poured into a sealed autoclave for solvothermal pretreatment at 80°C for 24 hours; subsequently, the resulting reaction solution was poured 0.7 g of polystyrene microspheres with a particle diameter of 200 nm were added to a petri dish, and after ultrasonication at 25 °C for 1 h, the solvent was volatilized at 60 °C for 24 h in an open state; finally, the obtained solid sample was roasted at 550 °C for 5 h, In addition to organic templates existing in mesoporous channels and polystyrene microspheres with macroporous structures, magnesium-aluminum composite oxides with highly ordered two-dimensional hexagonal mesoporous structures and network-like three-dimensional o...
Embodiment 2
[0047] Under vigorous stirring at 35°C, add 0.6 g of glacial acetic acid and 3.5 g of EO to 20 mL of absolute ethanol solution containing 1.8 g of 6M hydrochloric acid 106 PO 70 EO 106 , 1.0g magnesium nitrate hexahydrate and 3.95g aluminum tert-butoxide were completely dissolved to obtain a clear solution; the resulting clear solution was poured into a sealed high-pressure reactor, and solvothermal pretreatment was performed at 100°C for 24 hours; subsequently, the resulting reaction solution was poured into 0.7 g of polystyrene microspheres with a particle size of 200 nm were added to a petri dish, and after ultrasonication at 35 °C for 1 h, the solvent was volatilized at 60 °C for 24 h in an open state; finally, the obtained solid sample was roasted at 650 °C for 5 h, The magnesium-aluminum composite oxide sample is obtained by removing the organic template agent existing in the mesoporous channel and the polystyrene microspheres that produce a macroporous structure.
[0...
Embodiment 3
[0052] Under strong stirring at 40°C, add 0.4g oxalic acid and 2.0g EO to 20mL absolute ethanol solution containing 1.0g 6M nitric acid 20 PO 70 EO 20 , 0.1g of magnesium oxide and 6.0g of aluminum nitrate were completely dissolved to obtain a clear solution; the resulting clear solution was poured into a sealed autoclave, and solvothermal pretreatment was performed at 120°C for 24 hours; then, the resulting reaction solution was poured into a petri dish , adding 0.7g of polystyrene microspheres with a particle size of 300nm, ultrasonication at 40°C for 1h, and solvent volatilization at 80°C for 24h in an open state; finally, the obtained solid sample was roasted at 650°C for 5h to remove the The organic template agent in the pore channel and the polystyrene microspheres with macroporous structure were used to obtain the magnesium-aluminum composite oxide sample.
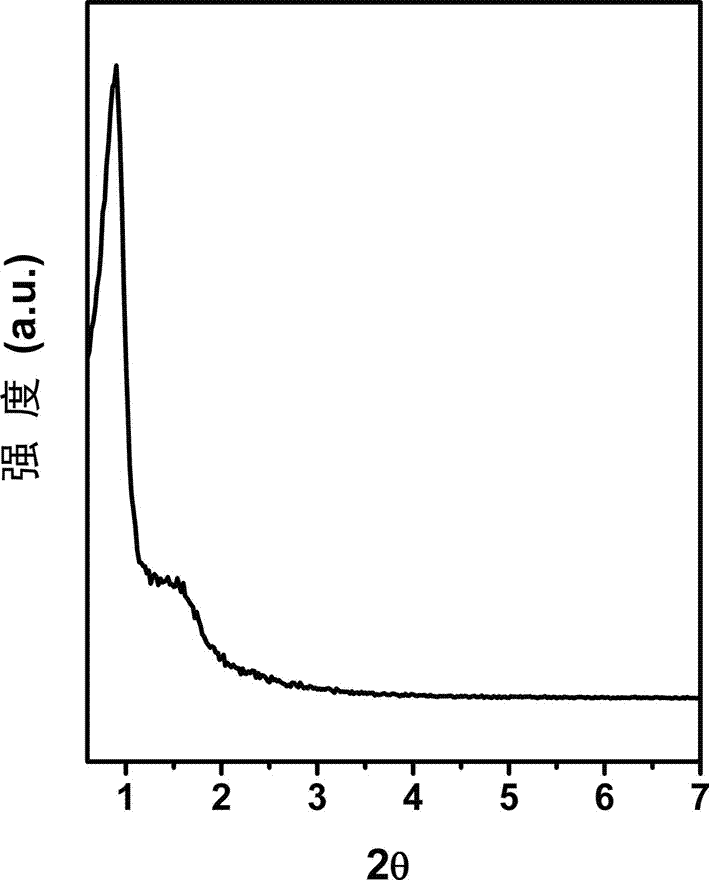
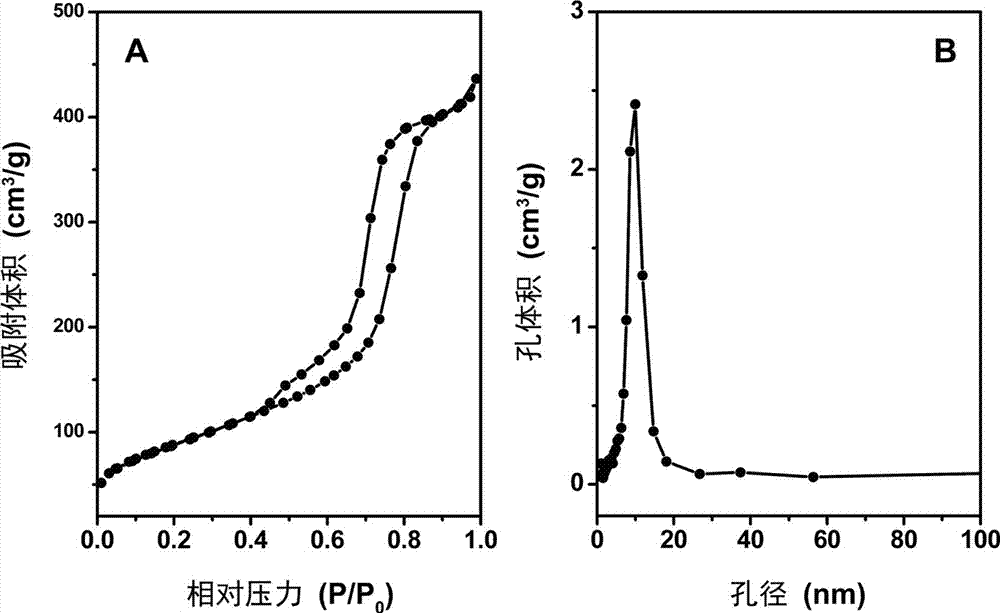
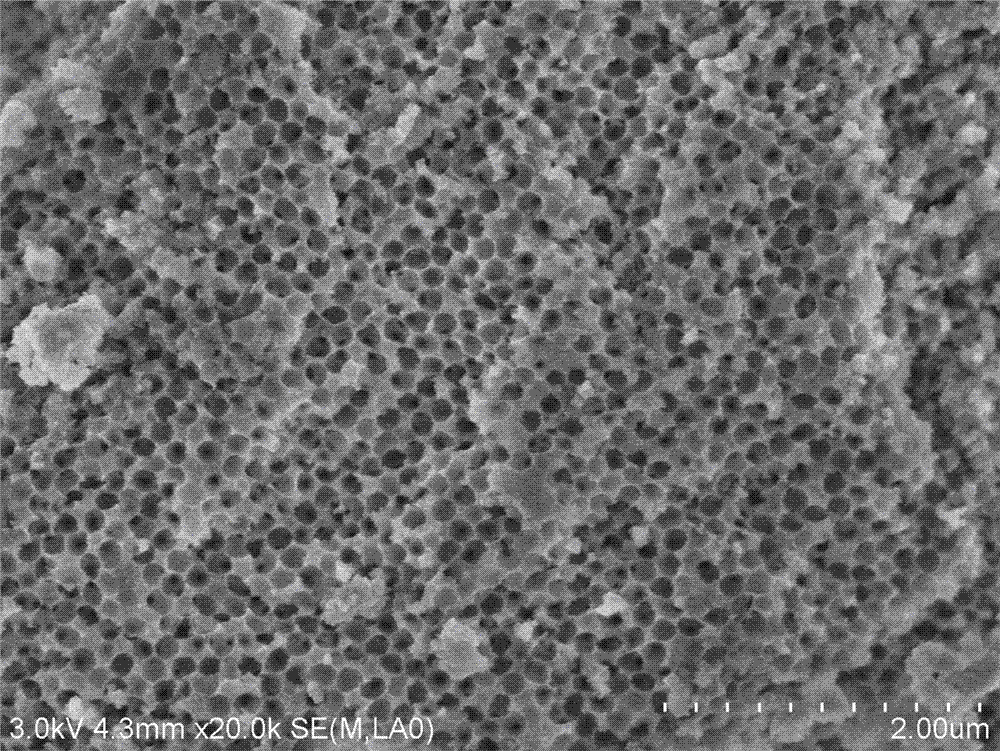
[0053] The XRD, SEM and nitrogen adsorption characterization results confirmed that the obtained magnesium-alum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Mesopore diameter | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com