Method for detecting thymine DNA glycosylase activity based on cyclophorase-remediation mediated double-signal amplification strategy
A dual-signal amplification, thymine technology, applied in the field of biological analysis, can solve problems such as limited sensitivity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Preparation of circular template DNA: Dilute the ligation probe and linear padlock probe with 1× tris(hydroxymethyl)aminomethane-ethylenediaminetetraacetic acid (Tris-EDTA) buffer to 10 micromoles per liter, and Denaturation at 95 degrees Celsius for 5 minutes. Then add 2 microliters of ligation probe and linear lock probe to 20 microliters of ligation buffer, including 1×T4 ligase buffer (6.6 millimoles per liter of magnesium chloride, 10 millimoles per liter of magnesium chloride). Dithiothreitol, 0.1 millimole per liter of adenosine triphosphate, 66 millimole per liter of tris(hydroxymethyl)aminomethane-hydrochloric acid (Tris-HCl) (pH 7.6)), 50 units of T4DNA ligase, in Incubate overnight at 16 degrees Celsius. After the ligation reaction, transfer 10 microliters of the ligation product to 10 microliters of digestion buffer, including 1 mmol per liter of dithiothreitol, 6.7 mmol per liter of magnesium chloride, and 67 mmol per liter of glycine -Potassium hydroxide ...
Embodiment 2
[0063] Cell lysis buffer preparation: 10 millimoles per liter of tris(hydroxymethyl)aminomethane-hydrochloric acid (Tris-HCl) (pH 8.0), 150 millimoles per liter of sodium chloride, 1% (mass / volume) Ethyl phenyl polyethylene glycol (NP-40), 0.25 millimoles per liter of sodium deoxycholate, 1% (mass / volume) glycerol, 0.1 millimoles per liter of 4-(2-aminoethyl) ) Benzenesulfonyl fluoride hydrochloride.
[0064] Cell extract preparation: The culture medium for human cervical cancer cells (HeLa) and human breast cancer cells (MCF-7) is Dulbec's modified Eagle with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin DMEM, placed in an incubator containing 5% carbon dioxide and 37 degrees Celsius for cultivation. When the cells grow to the logarithmic growth phase, they are digested with trypsin, and ice phosphate buffer (137 mmol sodium chloride solution, 2.7 mmol potassium chloride solution, 10 mmol phosphate buffer, pH 7.4) Wash twice, then centrifuge at 800 rpm at 4°C. T...
Embodiment 3
[0070] 3.1 Experimental verification of the principle
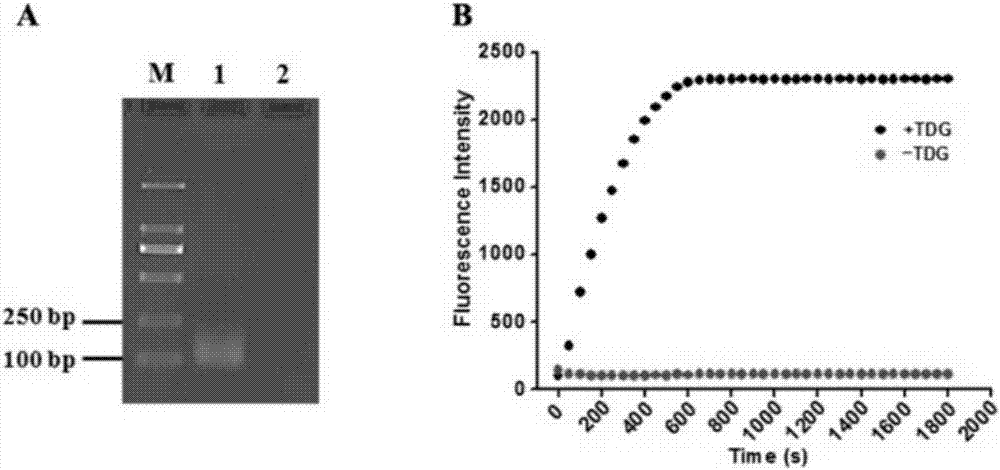
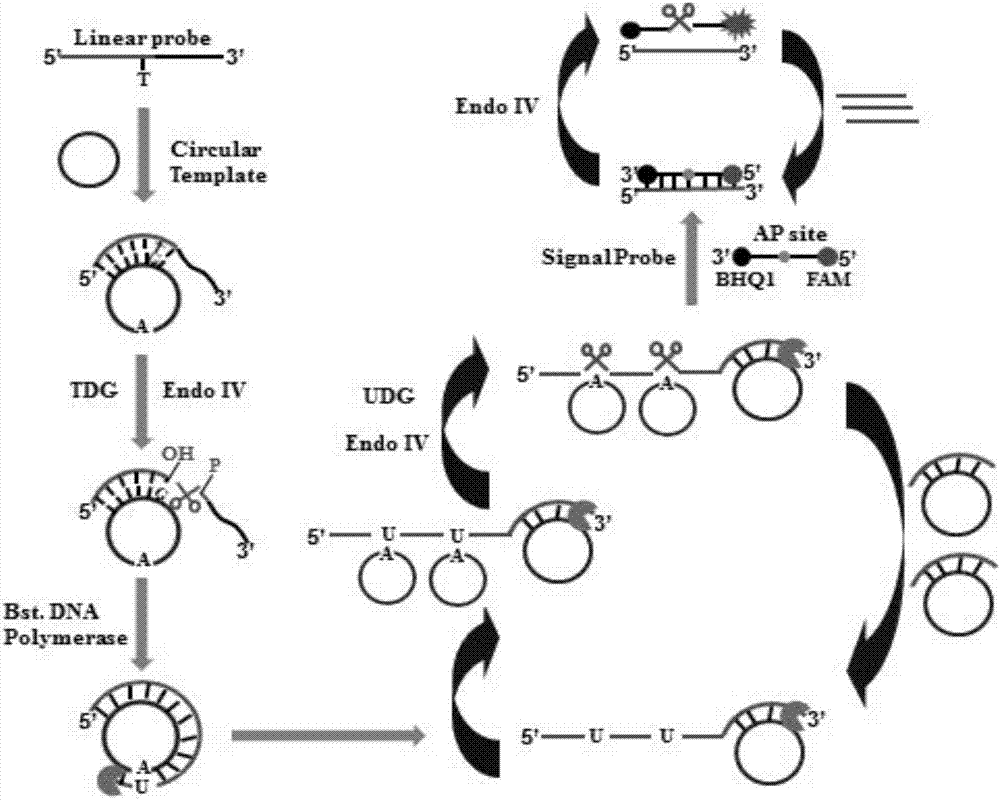
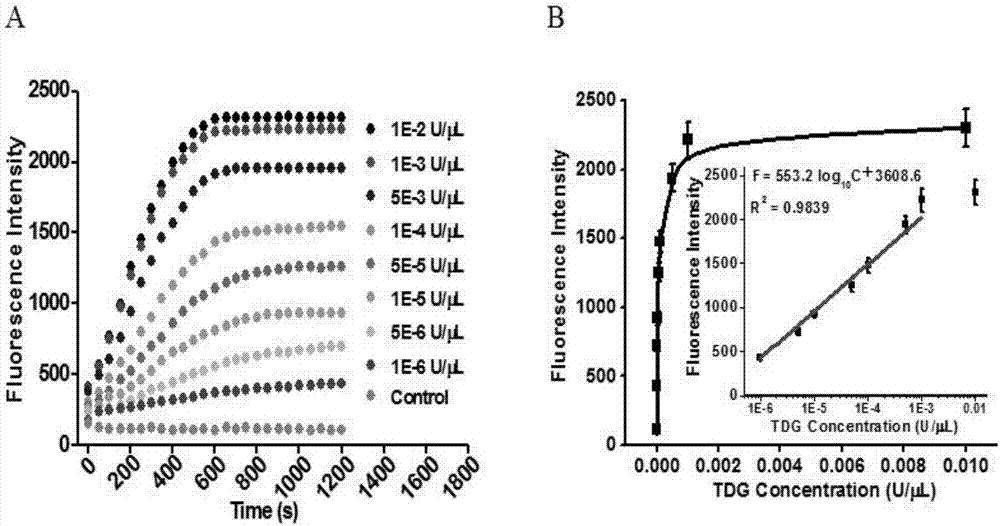
[0071] In order to verify the feasibility of this scheme, we tested and analyzed the reaction products, using 1% agarose gel electrophoresis with SYBR Gold as an indicator for verification and analysis. From figure 2 (A) It can be seen that when thymine DNA glycosylase (TDG) is present, characteristic bands of the reaction product can be seen, and when thymine DNA glycosylase (TDG) is not added, no characteristic bands appear. This is because thymine DNA glycosylase (TDG) can excise mismatched thymines and initiate subsequent uracil excision-mediated circular rolling circle exponential amplification. In order to prove the double-signal amplification reaction mediated by enzyme repair, we added a signal probe to detect the reaction process in real time with fluorescence. Such as figure 2 As shown in (B), in the presence of thymine DNA glycosylase (TDG), the fluorescence intensity increased rapidly 10 minutes before the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com