Preparation method of two-dimensional carbon-rich material rich in carbonyl, thioketone and selenone functional groups and application of two-dimensional carbon-rich material
A two-dimensional material, selenosulfone group technology, applied in electrical components, circuits, battery electrodes, etc., can solve the problem of difficult to achieve quantitative and accurate position, single type, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
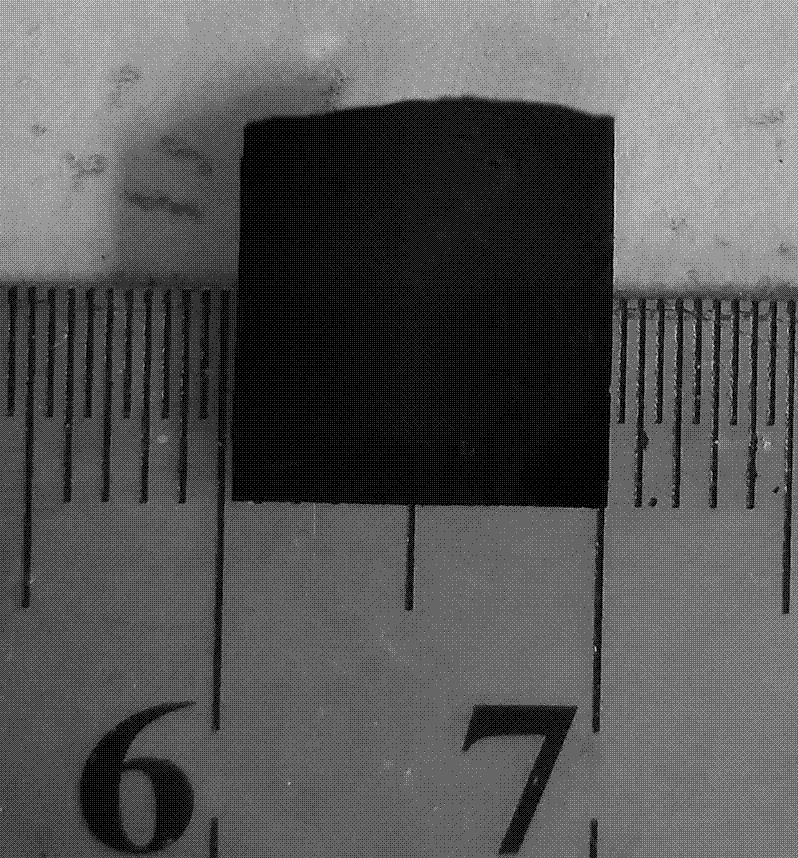
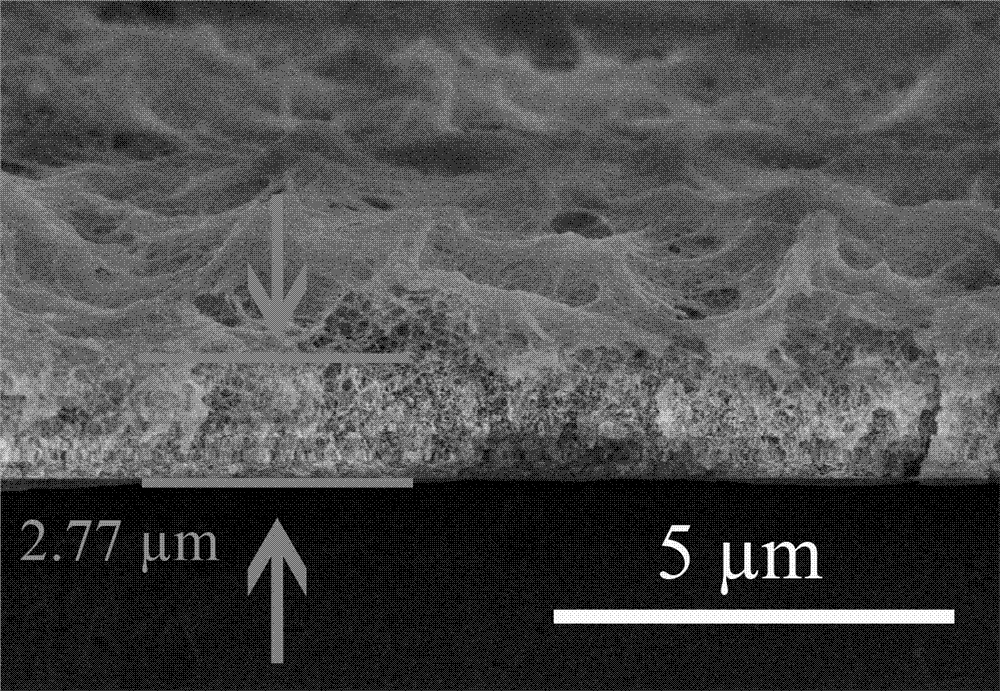
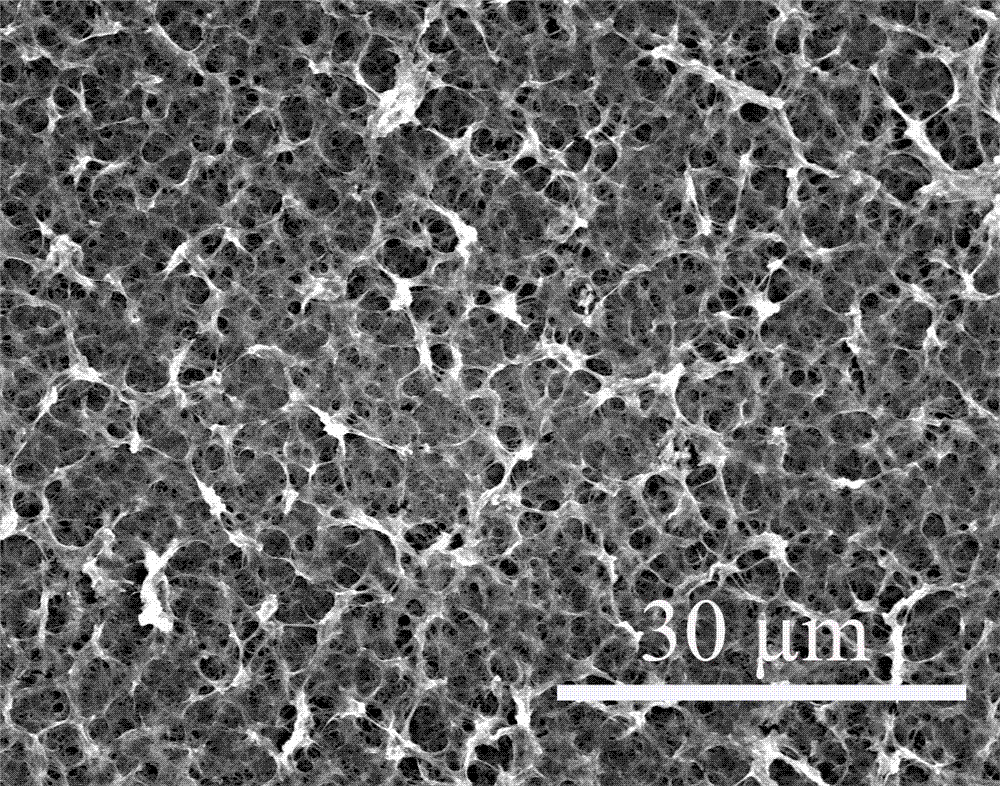
[0029]Under low temperature conditions, add 0.5 mL tetrabutylammonium fluoride (TBAF) (1 mol / L tetrahydrofuran solution, 0.5 mmol) to a tetrahydrofuran (THF) solution containing 40 mg (0.108 mmol) of compound 2, under argon protection The reaction was stirred for 5 minutes. The subsequent reaction solution was extracted three times with ethyl acetate and saturated brine, the organic phases were combined, dried over anhydrous sodium sulfate, and the organic solvent was removed by rotary evaporation at 30°C to obtain (monomer compound) (13 mg, 79%). Dissolved with 25 mL of dioxane, and slowly added dropwise under the protection of argon to the mixed solution containing 25 mL of dioxane, TMEDA and pyridine and 80 cm 2 In the two-neck bottle with copper foil catalyst base, the dropping time is 1 - 4 h. The reaction temperature is 60 o C Argon atmosphere for 3 days. After the reaction, a layer of light yellow film can be formed on the copper sheet, which is treated by FeCl 3 Th...
Embodiment 2
[0039] Under low temperature conditions, add 1.0 mL tetrabutylammonium fluoride (TBAF) (1 mol / L THF solution, 1.0 mmol) to a tetrahydrofuran (THF) solution containing 80 mg (0.216 mmol) of compound 3, under argon protection The reaction was stirred for 10 minutes. The subsequent reaction solution was extracted three times with ethyl acetate and saturated brine, the organic phases were combined, dried over anhydrous sodium sulfate, and the organic solvent was removed by rotary evaporation at 30 °C to obtain a tetrakynyl monomer (compound 5b) (25 mg, 76%). Dissolve it with 50 mL of dioxane, and slowly add it dropwise under the protection of argon to the mixed solution containing 50 mL of dioxane, TMEDA and pyridine and 150 cm 2 In the two-neck bottle with copper foil catalyst base, the dropping time is 2-4 h. The reaction temperature is 60 o C, reaction in argon atmosphere for 3 days. After the reaction, a layer of light yellow film can be formed on the copper sheet, which i...
Embodiment 3
[0041] Under low temperature conditions, add 1.5 mL tetrabutylammonium fluoride (TBAF) (1 mol / L THF solution, 1.5 mmol) to a tetrahydrofuran (THF) solution containing 120 mg (0.324 mmol) of compound 4, under argon protection The reaction was stirred for 10 minutes. The subsequent reaction solution was extracted three times with ethyl acetate and saturated brine, the organic phases were combined, dried over anhydrous sodium sulfate, and the organic solvent was removed by rotary evaporation at 30°C to obtain compound 5c (35 mg, 71%). Dissolve it with 50 mL of dioxane, and slowly add it dropwise under the protection of argon to the mixed solution containing 75 mL of dioxane, TMEDA and pyridine and 200 cm 2 In the two-neck bottle with copper foil catalyst base, the dropping time is 2-4 h. The reaction temperature is 60 o C, reaction in argon atmosphere for 3 days. After the reaction, a layer of light yellow film can be formed on the copper sheet, which is treated by FeCl 3 The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com