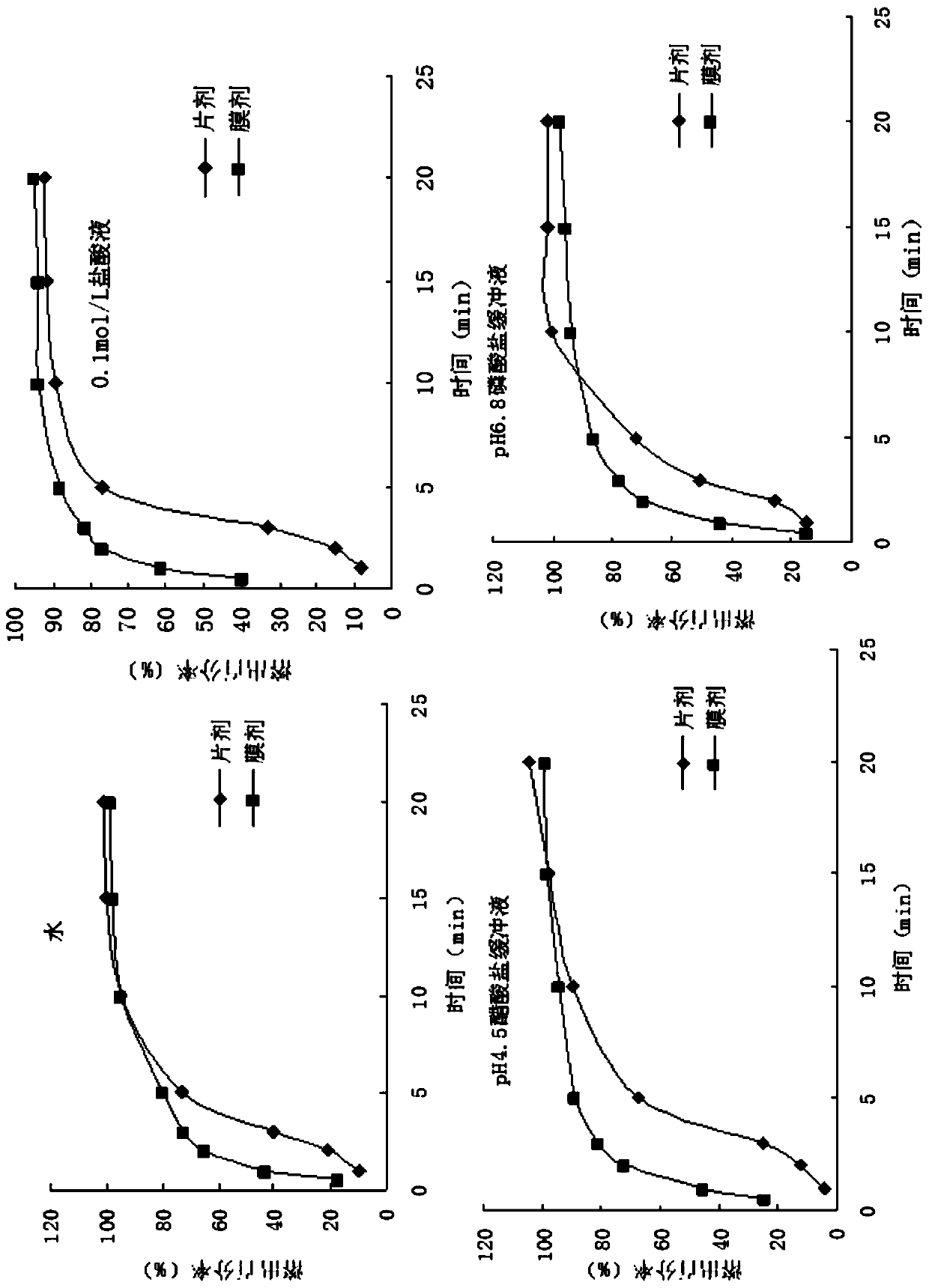
Risperidone fast-dissolving and/or fast-release solid oral film for gastrointestinal administration and preparation method thereof
A technology of risperidone and oral film, which is applied in the field of medicine, can solve the problems such as the technical solution of the preparation method, and achieve the effects of short disintegration time limit, fast dissolution rate and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
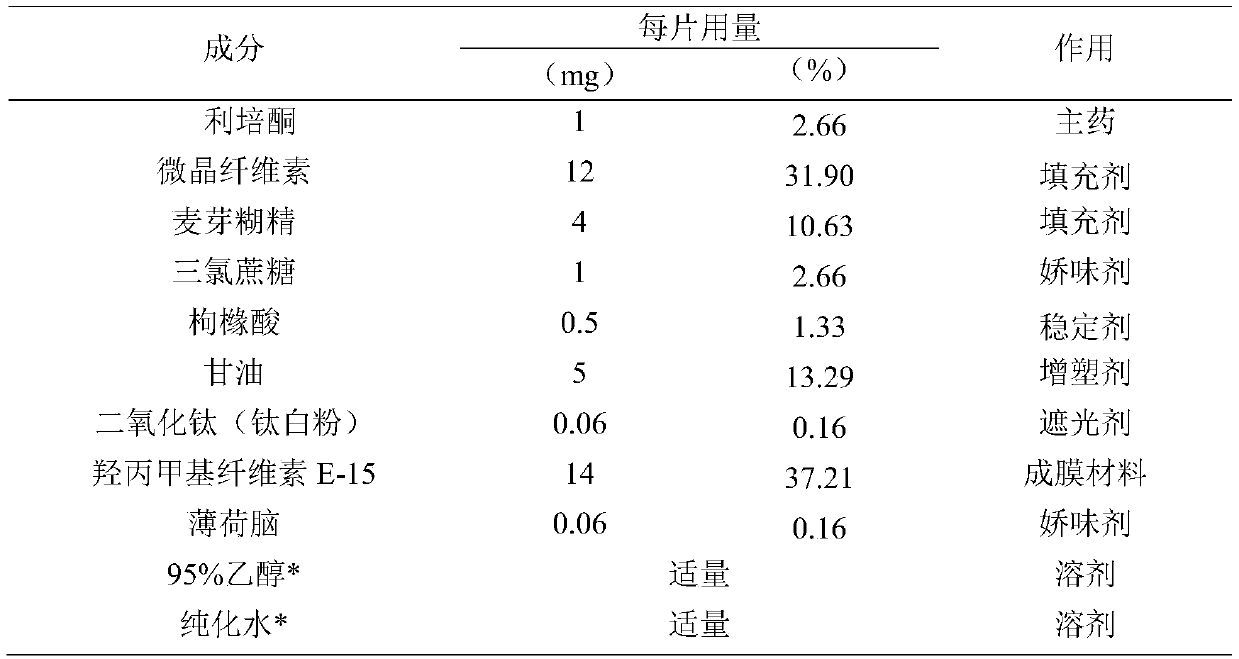
[0043]
[0044] *95% ethanol and purified water are removed after the membrane is dried.
[0045] Preparation method: all auxiliary materials such as risperidone raw material medicine, microcrystalline cellulose, maltodextrin, sucralose, etc. are passed through an 80-mesh sieve for use.
[0046] Weigh risperidone raw materials, microcrystalline cellulose, maltodextrin, titanium dioxide, sucralose, citric acid, hydroxypropyl methylcellulose (E-15), menthol, and glycerin according to the prescription ratio, and add 95% Use proper amount of ethanol and purified water, grind and stir to make the mixture evenly, set aside to defoam and set aside.
[0047] Adjust the running speed and height of the coating screw, the thickness of the coating film is about 100μm, so that the coating is continuous. Dry at 70°C for 1 hour to obtain.
Embodiment 2
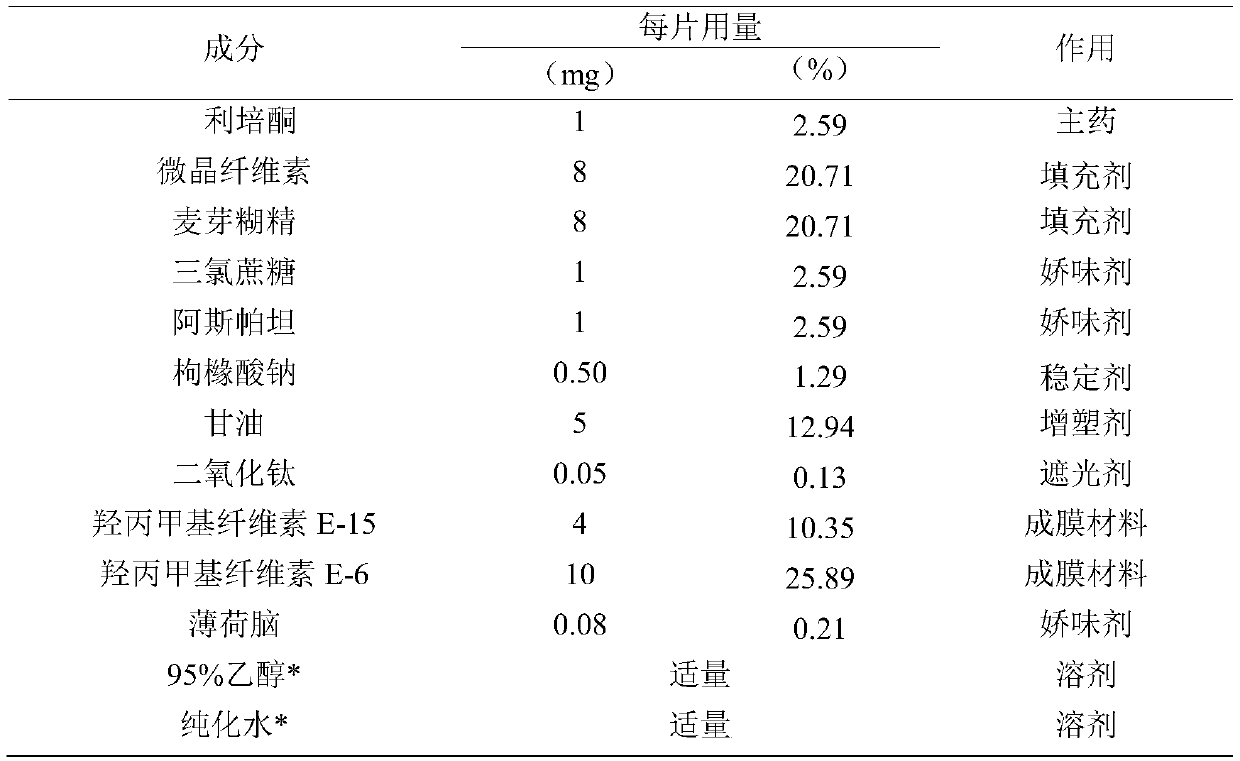
[0049]
[0050] *95% ethanol and purified water are removed after the membrane is dried.
[0051] Preparation method: all auxiliary materials such as risperidone crude drug, microcrystalline cellulose, maltodextrin, sucralose, aspartame, etc. are passed through an 80-mesh sieve for use.
[0052] Weigh risperidone raw materials, microcrystalline cellulose, maltodextrin, titanium dioxide, sucralose, aspartame, sodium citrate, hydroxypropyl methylcellulose (E-15), and hydroxypropyl according to the prescription ratio Methyl cellulose E-6, menthol, glycerin, add 95% ethanol and an appropriate amount of purified water, grind and stir to make the mixture uniform, stand for defoaming, and set aside.
[0053] Adjust the running speed and height of the coating screw, the thickness of the coating film is about 90μm, so that the coating is continuous. The drying temperature is 80°C and the drying time is 0.7 hours.
Embodiment 3
[0055]
[0056]
[0057] *95% ethanol and purified water are removed after the membrane is dried.
[0058] Preparation method: all auxiliary materials such as risperidone raw material medicine, microcrystalline cellulose, maltodextrin, aspartame, etc. are passed through an 80-mesh sieve for use.
[0059] Weigh risperidone raw materials, microcrystalline cellulose, maltodextrin, titanium dioxide, aspartame, citric acid, hydroxypropyl methylcellulose E-50, menthol, glycerin according to the prescription ratio, and add 95% ethanol And an appropriate amount of purified water, grind and stir to mix evenly, stand still to defoam, and set aside.
[0060] Adjust the running speed and height of the coating screw, the thickness of the coating film is about 100μm, so that the coating is continuous. Dry at 85°C for 0.5 hours to get.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com