Soluble expression increased bacillus pumilus W3 CotA laccase composite mutant
A technology of bacillus pumilus and mutants, applied in the field of bioengineering, can solve problems such as poor effect and secondary pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Construction of B. pumilus W3 laccase mutant
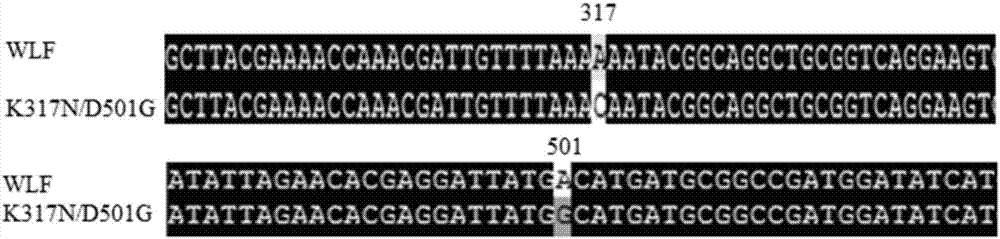
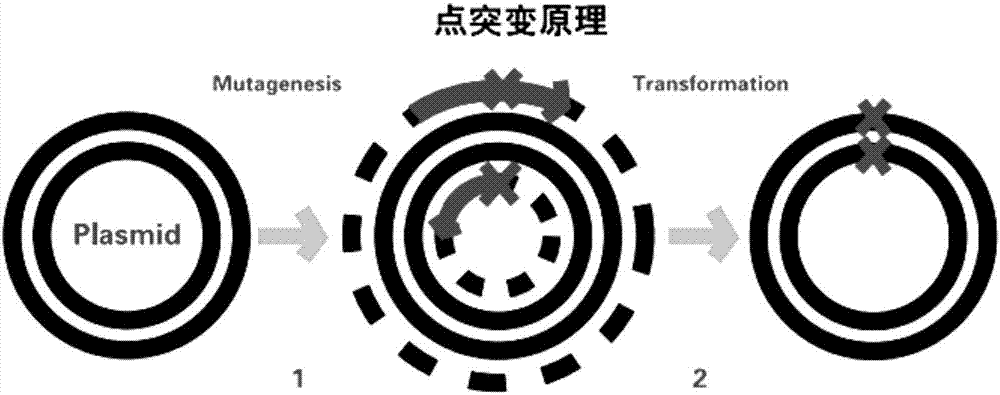
[0026] Principle of site-directed mutagenesis: The principle of point mutation uses primers to introduce mutation sites, and PCR SuperMix synthesizes mutant chains ( figure 2 ). Using the CotA laccase gene sequence of the previously constructed recombinant laccase mutant WLF as a template, primers were designed to mutate the 501st aspartic acid (Asp, D) in the laccase to glycine (Gly, D), and the mutation was successful Afterwards, position 317 was mutated with the D501G / WLF position template. The relevant forward primers and reverse primers designed are as follows:
[0027] 501 F5'-AACACGAGGATTAT GGC ATGATGCGGCC-3'
[0028] R5'- CC ATAATCCTCGTGTTCTAATATGTGAC-3'
[0029] 317 bit F5'-AACGATTGTTTTA AAC AATAAGGCAGGC-3'
[0030] R5'- GTT TAAAACAATCGTTTGGTTTTCGTAA-3'
[0031] The underlined parts represent the codons corresponding to the amino acids encoded by the mutant genes. The PCR amplification sys...
Embodiment 2
[0032] Example 2 Expression and purification of B.pumilus W3 laccase
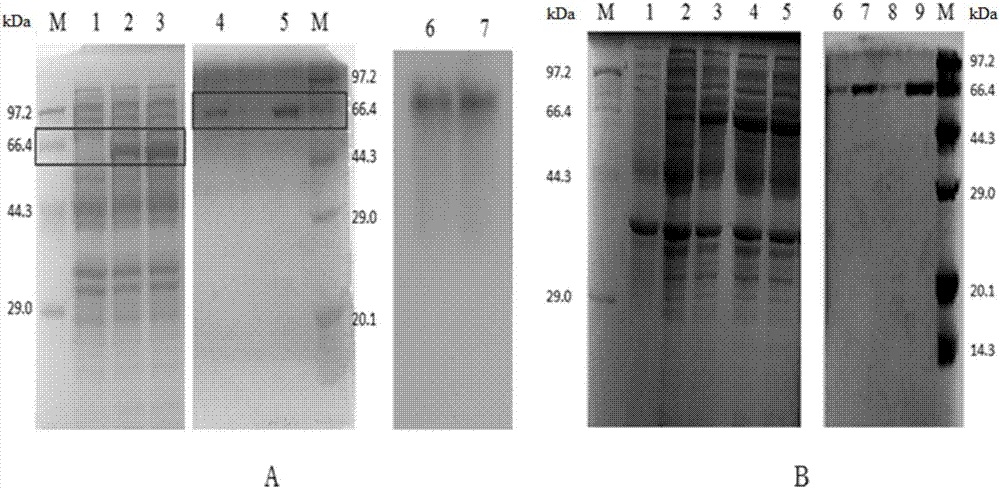
[0033] Expression: Inoculate wild-type recombinant laccase expression strains and previously constructed WLF and K317N / D501G / WLF recombinant expression strains from glycerol tubes into LB medium for activation, 37°C, 200rpm overnight (10h). Insert the seeds into 50mL LB liquid fermentation medium (containing 100mg L -1 Ampicillin) 37 ℃ 200rpm shaker culture to OD 600 After reaching 0.5, the shaker temperature was adjusted to 15°C for static culture for 30 minutes, and then the final concentration of 0.4mM IPTG and 0.25mM CuSO was added 4 For induction, culture at 200 rpm at 15°C for 24 hours, centrifuge the fermentation broth at 8000 rpm at 4°C for 10 minutes to remove the supernatant, and collect the cells. Resuspend the collected bacteria with phosphate buffer, and after resuspension, use an ultrasonic cell disruptor to crush the bacteria to release intracellular proteins. The supernatant was heated in...
Embodiment 3
[0035] Example 3 Enzyme activity determination and expression analysis of B.pumilus W3 laccase
[0036] (1) Definition of enzyme activity unit: When using the ABTS method to determine the enzyme activity of laccase, define the amount of enzyme required to catalyze the conversion of 1 μmol of substrate into product per minute as an activity unit.
[0037] (2) Enzyme activity determination steps: 1 Preheating: Take 2.4mL of citric acid buffer solution with pH 4.0 in a test tube, add 0.5mL ABTS solution (the final concentration of ABTS is 0.5mM) into the test tube and place it in a water bath at 50°C for preheating. Heat for 5 minutes; 2 reactions: add diluted 0.1mL sample enzyme solution, shake evenly. 3 Measurement: Use a spectrophotometer to measure the kinetics of the uniformly oscillating sample, measure the change in OD value per minute within 30s at a wavelength of 420nm (the measurement reaction shows a straight line) and calculate the enzyme activity.
[0038] (3) Deter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com