Synthesis method of trifluoropyruvate compounds
A technology of trifluoropyruvate and trifluoropropionate, which is applied in the field of synthesis of trifluoropyruvate compounds, can solve the problems of complex preparation process, complex process and long reaction time of solid acid catalysts, and achieve catalyst Reusable, simple process route, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A kind of synthetic method of trifluoropyruvate compounds proposed by the present invention comprises the following steps:
[0022] S1. Preparation of new solid acid catalyst: Fill 100ml of alumina particles with a particle size of 50 μm in a vertical tubular reactor equipped with a split tubular reactor, and conduct the alumina particles at 200°C under a nitrogen atmosphere. Fully dry, the drying time is 12h, and then at 200°C, pass through R22 to activate the alumina particles, the activation time is 12h;
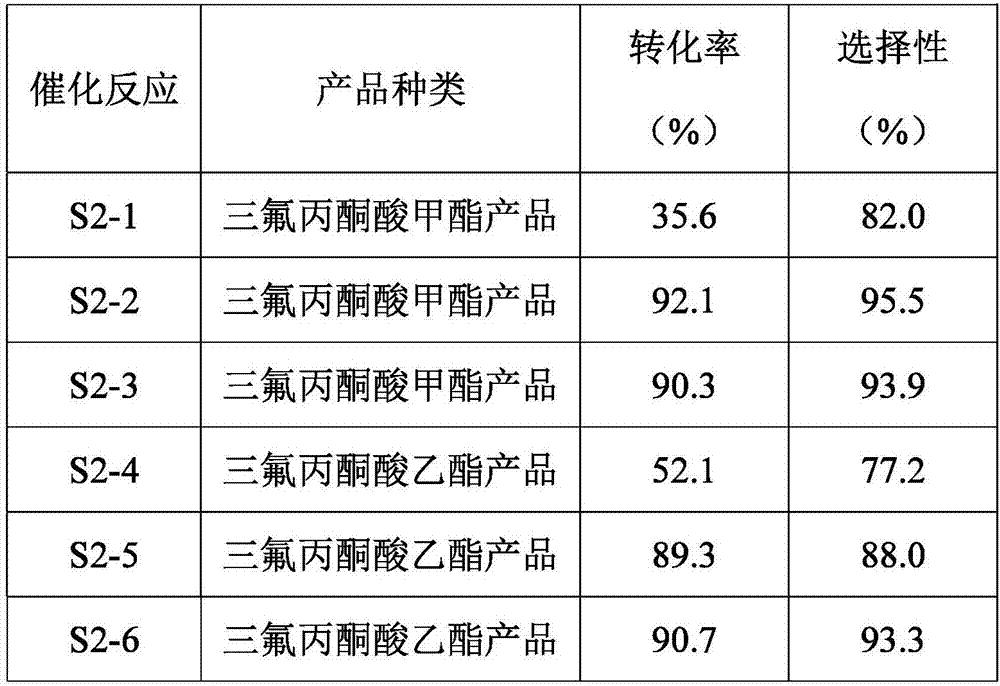
[0023] S2-1. Gas-phase catalytic reaction: gasify 2-fluoro-2-methoxy-trifluoropropionic acid methyl ester and pass it into a tubular reactor with the new solid acid catalyst synthesized in step S1 at 140°C, React under the condition of feeding nitrogen, control the residence time of 2-fluoro-2-methoxyl-trifluoropropionic acid methyl ester in the tubular reactor to be 10s, pass the reacted gas into the tank placed in the ice-water bath Receive the sample in the bot...
Embodiment 2
[0028] A kind of synthetic method of trifluoropyruvate compounds proposed by the present invention comprises the following steps:
[0029] S1. Preparation of a new solid acid catalyst: Fill 100ml of alumina particles with a particle size of 5000 μm into a vertical tubular reactor equipped with a split tubular reactor. Fully dry, the drying time is 12h, and then at 300°C, pass through R12 to activate the alumina particles, the activation time is 12h;
[0030] S2-4. Gas-phase catalytic reaction: gasify ethyl 2-fluoro-2-ethoxy-trifluoropropionate and pass it into a tubular reactor with the new solid acid catalyst synthesized in step S1 at 150°C, React under the condition of feeding nitrogen, control the residence time of 2-fluoro-2-ethoxyl-trifluoropropionic acid ethyl ester in the tubular reactor to be 20s, pass the gas after the reaction into the tank placed in the ice-water bath Receive the sample in the bottle, weigh and analyze the sample to obtain the crude product of ethy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com