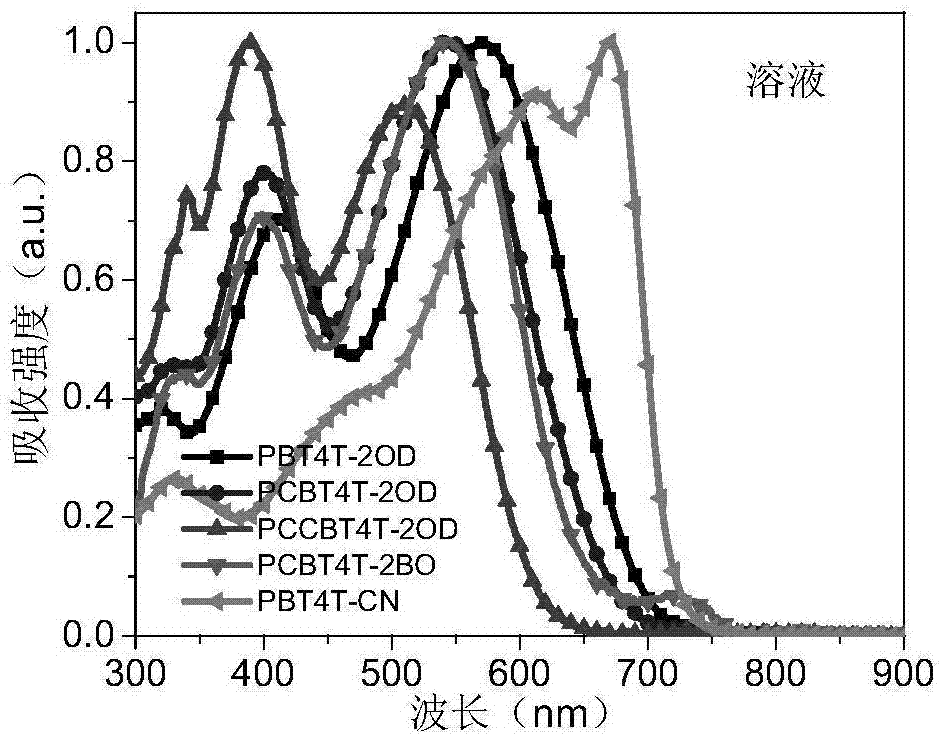
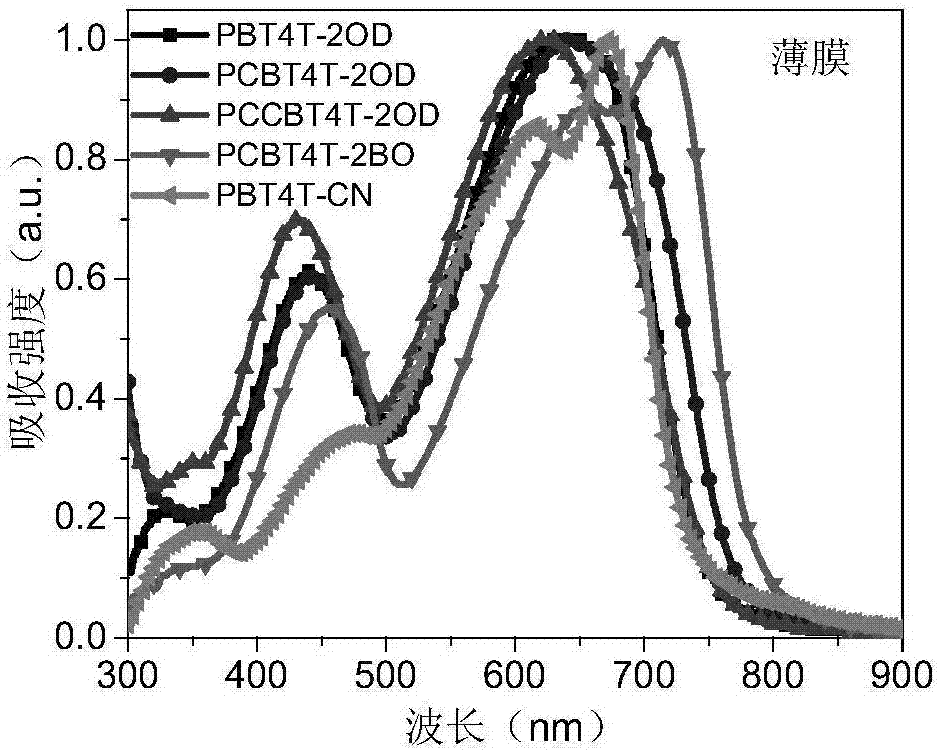
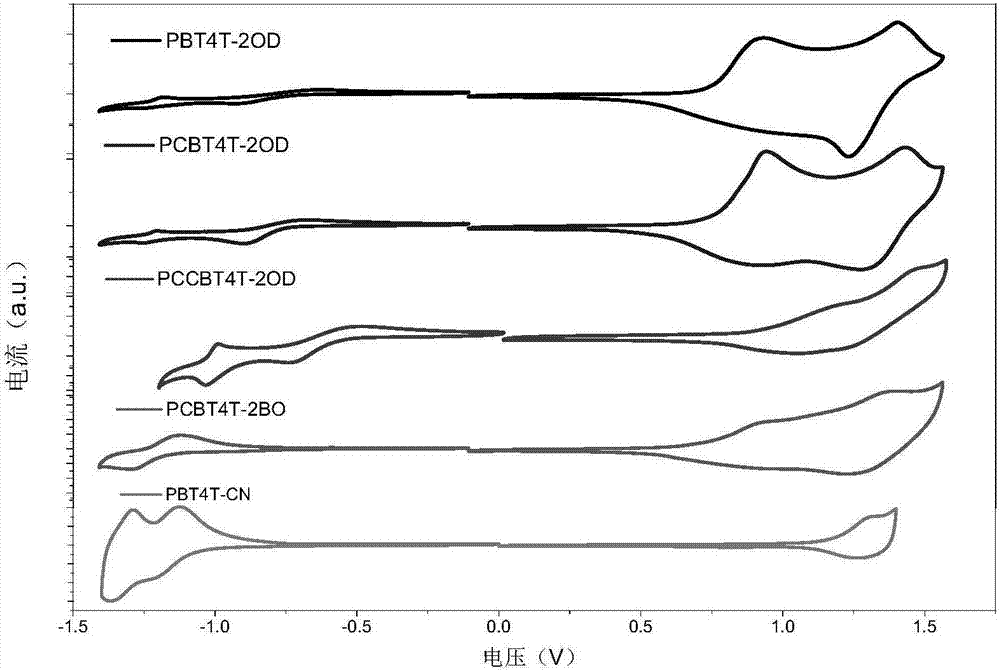
Exotic atom-substituted benzothiadiazole-based polymer donor material and preparation method and application thereof
A technology of benzothiadiazole and polymer, which is applied in the field of benzothiadiazole-based polymer donor materials and its preparation, can solve the problems of toxic fluorine reagents, cumbersome synthesis steps, and low yield, and achieve spatial structure Optimizing and improving the effect of photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] In this example, the polymer PCBT4T-2OD was prepared by the following method:
[0107] (1) 5-chloro-4,7-bis(5-bromo-4-(2-octyldodecyl)-2-thiophene)-2,1,3-benzothiadiazole (CBT2T-2OD )Synthesis.
[0108]
[0109] ①5-Chloro[2,1,3]benzothiadiazole (2): Add 5-chloro-1,2-o-phenylenediamine (7.83g, 55mmol), pyridine (13.2ml, 165mmol) and chloroform (600ml). Thionyl chloride (8ml, 110mmol) was then added dropwise to the reaction solution at room temperature using a syringe. Subsequently, the reaction was heated to 65 °C and stirred overnight. The reaction was stopped, and after cooling to room temperature, the reactant was quenched by pouring into 200ml of dilute hydrochloric acid, and extracted three times with chloroform. The organic phases were combined, washed with saturated brine, dried over anhydrous magnesium sulfate, filtered and spin-dried, and the residual solid was purified by column chromatography (petroleum ether: dichloromethane, 10:1) to obtain 5.96 g of ...
Embodiment 2
[0122] In this example, polymer PCCBT4T-2OD was prepared by the following method:
[0123] (1) 5,6-dichloro-4,7-bis(5-bromo-4-(2-octyldodecyl)-2-thiophene)-2,1,3-benzothiadiazole ( Synthesis of CCBT2T-2OD).
[0124]
[0125]①5,6-dichloro[2,1,3]benzothiadiazole (6): Add 5,6-dichloro-1,2-o-phenylenediamine (5g , 28.24mmol), pyridine (6.82ml, 84.72mmol) and chloroform (500ml). Thionyl chloride (5.13ml, 70.6mmol) was then added dropwise to the reaction solution at room temperature using a syringe. Then heat up to 65°C under reflux and stir overnight, take a sample on the next day, and stop heating after confirming that the reaction is complete. After cooling to room temperature, pour into 200ml of dilute hydrochloric acid to quench, and extract three times with chloroform. The organic phases were combined, washed three times with saturated brine, dried over anhydrous magnesium sulfate, filtered and spin-dried, and purified by column chromatography (petroleum ether:dichlorome...
Embodiment 3
[0134] In this example, the polymer PCBT4T-2BO was prepared by the following method:
[0135] (1) Synthesis and examples of 5-chloro-4,7-bis(5-bromo-4-(2-hexyldecyl)-2-thiophene)-2,1,3-benzothiadiazole 1 The difference in step (1) is that 2-tributyltin-4-(2-octyldodecyl)thiophene in step ③ is replaced by 2-tributyltin-4-(2-hexyldecyl)thiophene, Thus, 5-chloro-4,7-bis(5-bromo-4-(2-hexyldecyl)-2-thiophene)-2,1,3-benzothiadiazole was prepared.
[0136] (2) The synthesis of 3-cyano-5,5'bis(trimethylstannyl)-2,2'bithiophene is the same as in Example 1.
[0137] (3) Synthesis of polymer PCBT4T-2BO, the chemical reaction formula is as follows:
[0138]
[0139] In a sealed 25ml tube, add 5-chloro-4,7-bis(5-bromo-4-(2-pentyldecyl)-2-thiophene)-2,1,3-benzothiadi Azole (121.3mg, 0.129mmol), 5,5'-bis(trimethyltinthiophene)-2,2'bidithiophene (63.4mg, 0.129mmol), Pd 2 (dba) 3 (4.76mg, 0.0052mmol) and P(o-tol)3 (6.40mg, 0.021mmol). After fully ventilating, 8ml of chlorobenzene was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com