Solid-state lithium battery polymer electrolyte and preparation method and application thereof
A technology of solid electrolytes and polymers, applied in solid electrolytes, lithium batteries, non-aqueous electrolytes, etc., can solve the problems of low room temperature ionic conductivity, low room temperature ionic conductivity, and difficulty in forming SEI films, etc., and achieve high ionic conductivity high efficiency, simple preparation process, and the effect of improving ionic conductivity at room temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
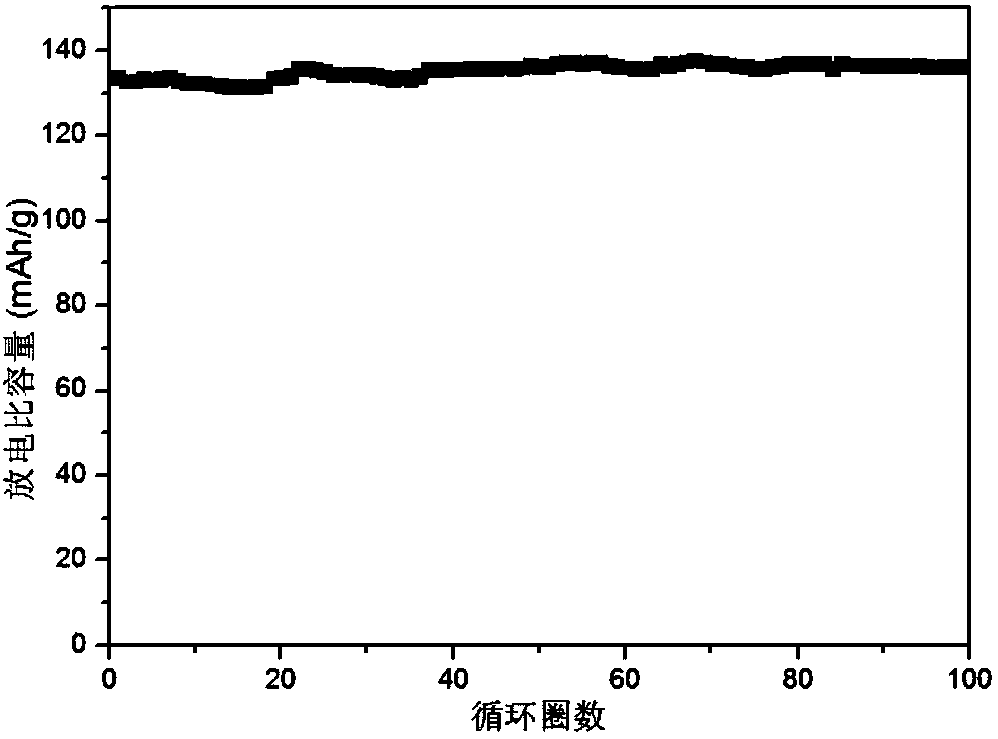
Embodiment 1
[0050] Li-ion polymer electrolyte
[0051] Polyethylene carbonate is obtained by ring-opening polymerization of carbon dioxide and propylene oxide, wherein the substance ratio of carbonate repeating unit to ethylene repeating unit is 1:1;
[0052] In the glove box, add 0.103g of trifluoroethanol to 2ml of ethylene glycol dimethyl ether solvent, add a magnet and stir to make the dispersion uniform, then add 0.24g of anhydrous lithium hydroxide to the above solution, stir to make the reaction complete, and then Add 0.142g of boron trifluoride ether solution to the above solution, volatilize the solvent under Ar gas condition, and vacuum dry at 60°C to remove the remaining solvent to obtain a dry white solid, and obtain the formula 1 where R is CF 3 CH 2 - Lithium trifluoroethoxytrifluoroborate.
[0053] Dissolve 1.0g of polyethylene carbonate and 0.2g of trifluoroethoxylithium trifluoroborate in 15ml of acetonitrile, stir at room temperature until it is in a homogeneous solution...
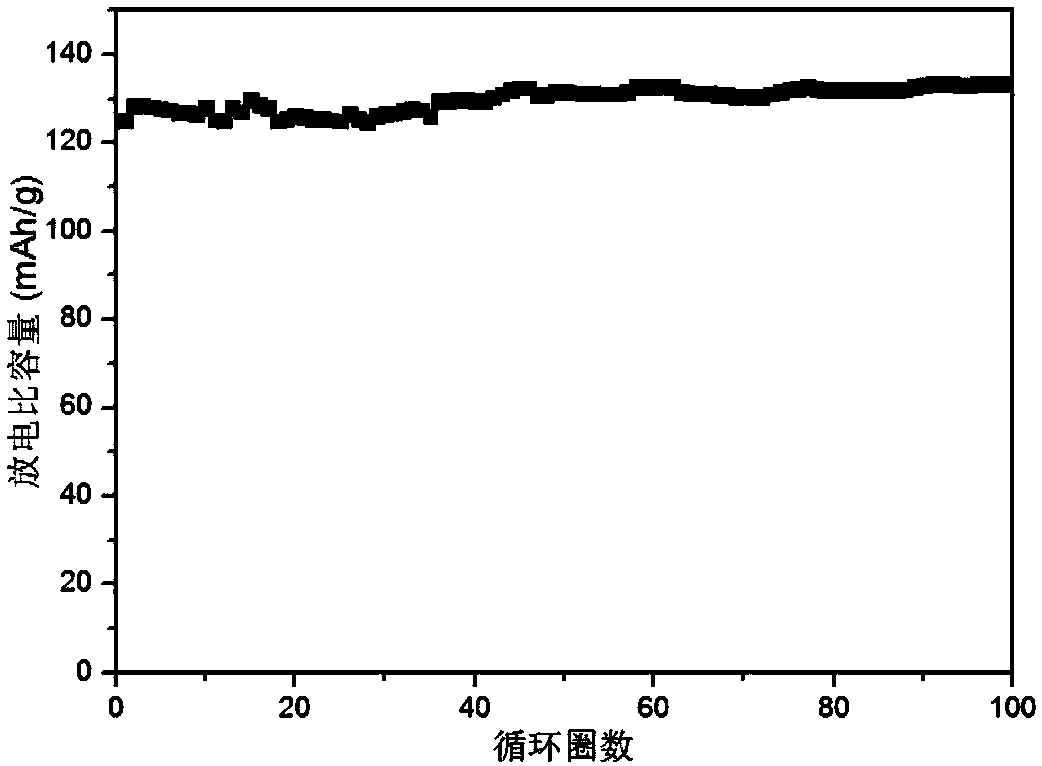
Embodiment 2
[0063] Li-ion polymer electrolyte
[0064] Polypropylene carbonate is obtained by ring-opening polymerization of carbon dioxide and propylene oxide, wherein the substance ratio of carbonate repeating unit to propylene repeating unit is 1:1;
[0065] In the glove box, 0.236g of perfluoro-tert-butanol was added to 2ml of ethylene glycol dimethyl ether solvent, and a magnet was added to stir to disperse evenly, and then 0.0625ml of butyllithium (1.6M in hexane) was added to the above solution, Stir to make the reaction complete, then add 0.142 g of boron trifluoride ether solution to the above solution, evaporate the solvent under Ar gas, and dry in vacuo at 60°C to remove the remaining solvent to obtain a dry white solid. where R is (CF 3 ) 3 C-perfluorotert-butoxytrifluoroborate lithium salt.
[0066] Dissolve 1.3g of polypropylene carbonate and 0.4g of perfluorotert-butoxy lithium trifluoroborate in 15g of N,N-dimethylformamide, stir at room temperature until it is in a hom...
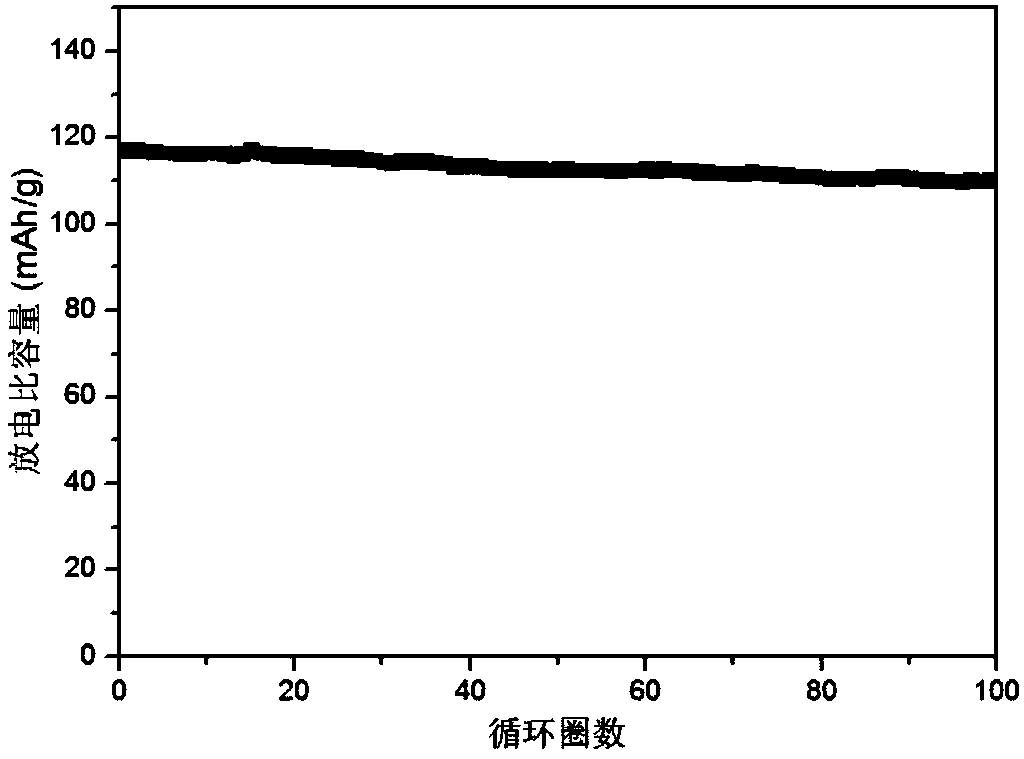
Embodiment 3
[0076] Li-ion polymer electrolyte
[0077] Polybutylene carbonate is obtained by ring-opening polymerization of carbon dioxide and 1,2-butylene oxide, wherein the ratio of carbonate repeating unit to butylene repeating unit is 1:1;
[0078] Dissolve 2g of polybutylene carbonate and 0.6g of perfluorotert-butoxylithium trifluoroborate in 20ml of tetrahydrofuran, stir at room temperature until it is in a homogeneous solution state, take 3g of the above solution on polyethylene terephthalate nonwoven Coated on a cloth (5cm*5cm), and dried the obtained polymer electrolyte in a vacuum oven at 70°C overnight. Cut to size.
[0079] Test the ionic conductivity of the lithium ion polymer electrolyte obtained above (perfluorotert-butoxy trifluoroborate lithium / polypropylene carbonate electrolyte): the polymer electrolyte is sandwiched between two pieces of stainless steel and placed in a 2032 battery in the shell. Ionic conductivity is measured by electrochemical AC impedance spectros...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com