Cardanol-based acrylate reactive diluent and preparation method and application thereof
A technology of cardanol-based acrylate and reactive diluent, which is applied in the fields of radiation-cured coatings, inks, and adhesives. It can solve the problems of large volume shrinkage and easy volatilization of reactive diluents, and achieve simple synthesis process and excellent dilution function. , reduce the effect of volatile substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
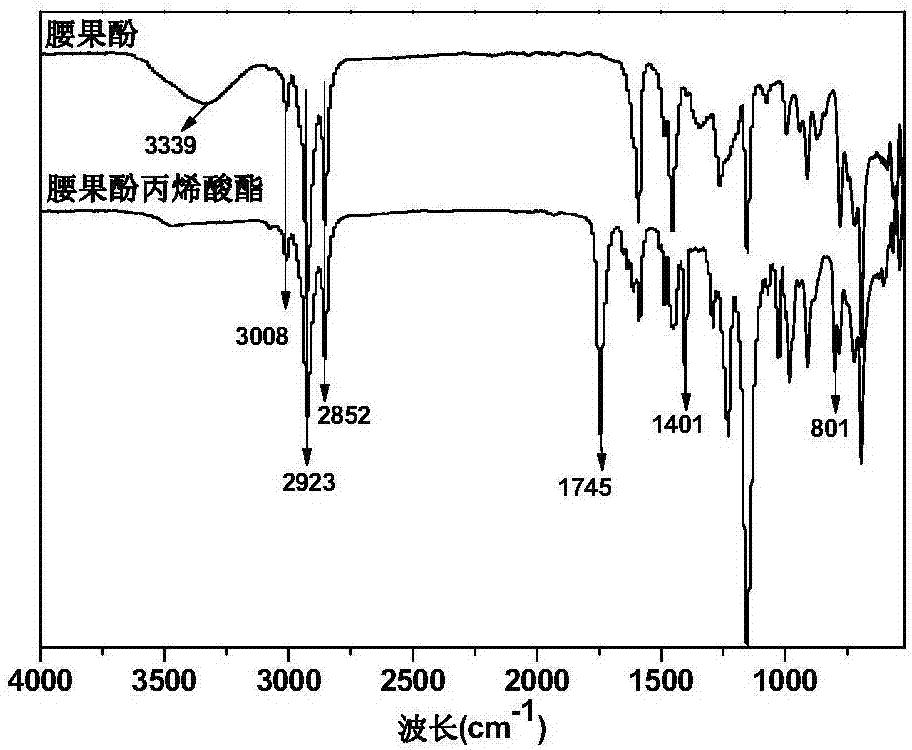
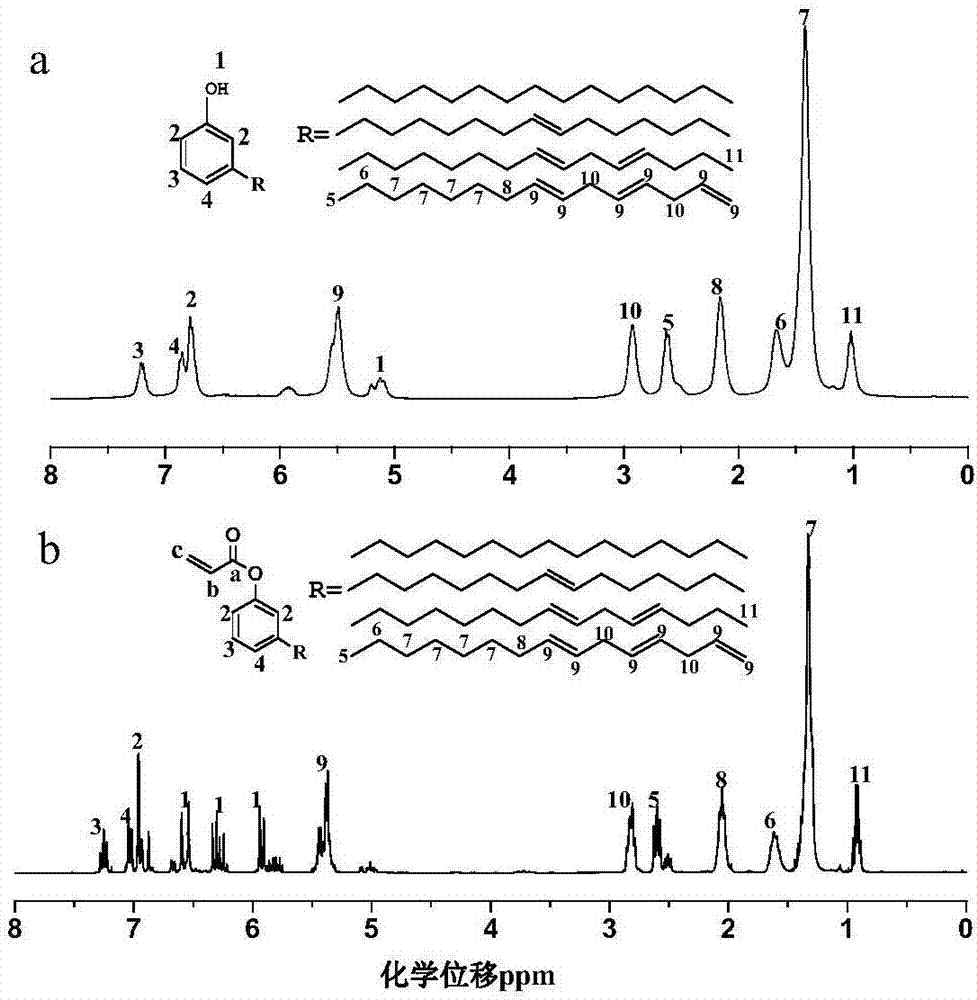
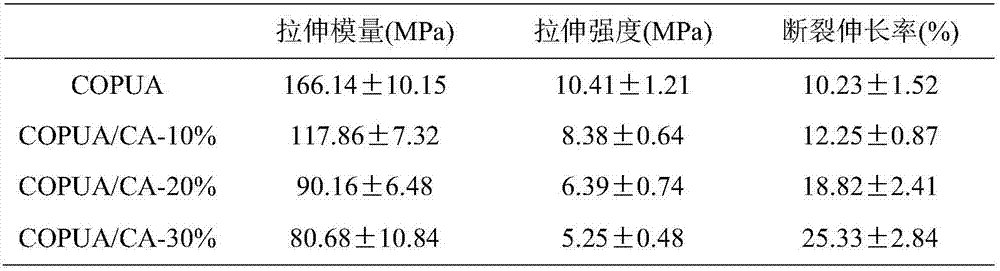
[0020] (1) Esterification reaction: add 0.1 mol of cardanol and 2wt.% of reactant sodium hydroxide as a catalyst to a four-necked flask equipped with a condenser, a stir bar and a thermometer, and then add 0.1 mol of acid binding agent hydrogen carbonate Sodium, 1wt.% of reactants, polymerization inhibitor hydroquinone, and 20mL solvent tetrahydrofuran, add 0.09mol acryloyl chloride dropwise under ice-water bath conditions, the dropwise addition is completed within 30min, the temperature is raised to 30℃ and react for 10 hours, and the precipitate is filtered The filtrate is subjected to a vacuum rotary evaporator to remove the solvent to obtain a crude product. (2) Post-treatment: The crude product is first extracted with ether, washed 3 times with saturated sodium bicarbonate aqueous solution, 3 times with ultrapure water, left to stand for layering, and then retain the organic layer, and then dried with anhydrous sodium sulfate and allowed to stand overnight. , Evaporate the...
Embodiment 2
[0022] (1) Esterification reaction: add 0.1 mol of cardanol and 2wt.% of the reactant, catalyst sodium bicarbonate, into a four-necked flask equipped with a condenser, stir bar and thermometer, and then add 0.11 mol of acid binding agent triethyl Amine, polymerization inhibitor p-hydroxyanisole, which accounts for 1wt.% of reactants, and 20mL solvent n-hexane, add 0.11 mol of acryloyl chloride dropwise under ice-water bath conditions, add dropwise within 30 minutes, raise the temperature to 50°C for 7 hours , The precipitate is filtered, and the filtrate is subjected to a vacuum rotary evaporator to remove the solvent to obtain a crude product. (2) Post-treatment: The crude product is first extracted with ether, washed 3 times with saturated sodium bicarbonate aqueous solution, 3 times with ultrapure water, left to stand for layering, and then retain the organic layer, and then dried with anhydrous sodium sulfate and allowed to stand overnight. , Evaporate the solvent to obtain...
Embodiment 3
[0024] (1) Esterification reaction: add 0.1 mol of cardanol and 2wt.% of reactant 4-dimethylaminopyridine as a catalyst, into a four-necked flask equipped with a condenser, a stirring rod and a thermometer, and then add 0.1 mol of bound acid Reagent triethylamine, polymerization inhibitor hydroquinone accounting for 1wt.% of the reactants, and 20mL solvent toluene, add 0.1 mol of acryloyl chloride dropwise under ice-water bath conditions, the dropwise addition is completed within 30 minutes, and the temperature is raised to 60°C for 8 hours , The precipitate is filtered, and the filtrate is subjected to a vacuum rotary evaporator to remove the solvent to obtain a crude product. (2) Post-treatment: The crude product is first extracted with dichloromethane, washed 3 times with saturated sodium bicarbonate aqueous solution, 3 times with ultrapure water, left to stand for layering, and then retain the organic layer, then dry with anhydrous sodium sulfate and stand still After one n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com